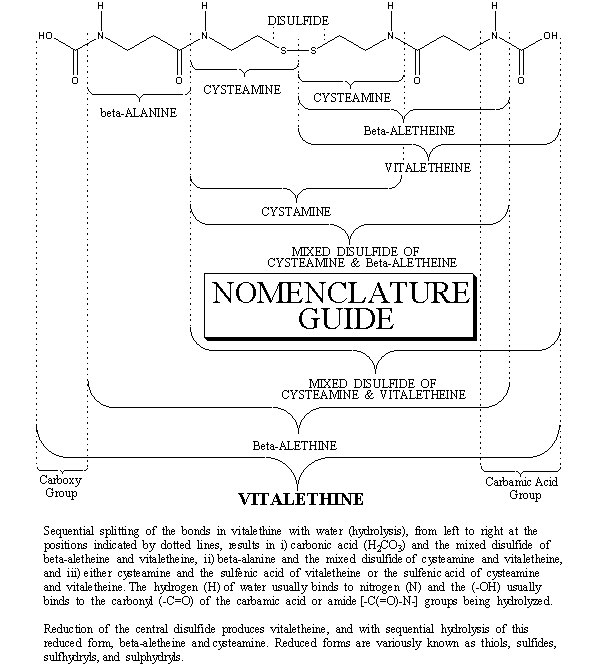
STRUCTURE OF VITALETHINE:
HYDROLYSIS, DECOMPOSITION,
REDUCTION

Exhaustive chemical and structural information for the vitaletheine modulators is available in the Journal section of this web site, under the article about the Pitfalls in the synthesis of the vitaletheine modulators. The sulfenate-linked benzyl carbamate dimer in its hydrated form is illustrated with the mass spectra proof of structure.
ATTEMPTS AT IUPAC NOMENCLATURE
Although the nomenclature, supra, is for the most part "trivial",
such conventions are necessary to make these compounds easily recognizable
for the general public. For example, one attempt at IUPAC nomenclature
for the simple thiol, vitaletheine is.....
N-[3-(2-mercapto-ethanamino)-3-oxo-3,1-propanediyl]-carbamic acid
Similarly derived, the disulfide, vitalethine, is....
3,3'-[dithiodi[(2,1-ethanediyl)-amino]]-bis-[N-(3-oxo-3,1-propanediyl)-carbamic acid]
Note that vitalethine is created by V-shaped additions to the beta-alethine molecule, each consisting of two oxygens and one carbon, the building block of plant life as we known it. In so doing, the biological potency, "Vita", of authentic preparations of "alethine" increases dramatically as "Vit"alethine is formed. Often this increase in potency for the authentic preparations is 100s of millions if not billions of times. The biological potency of others' contaminated preparations of beta-althine will probably increase trillions of times when converted to pure "vit"alethine.
Though interpretable by most chemists, the more biochemical and peptide-like trivial names for even the relatively simple thiol, beta-aletheine....
beta-alanyl-cysteamine
.....become much more complex when IUPAC nomenclature is attempted....
N-(2-mercaptoethane)-3-aminopropanamide
An attempt to name the disulfide of beta-aletheine, beta-alethine, is even more difficult to interpret for most people.....
N,N'-[dithiodi(2,1-ethanediyl)]-bis[3-aminopropanamide]
When one considers that a single error in hyphen, space, bracket, spelling, Greek letter, etc. could cause a computer or internet search routine for these IUPAC names to fail, thereby producing " no hits", the need for simpler, easily recognized naming conventions for common use becomes obvious. Unlike others' nomenclature proposals, these by the original discoverers of these compounds are based upon past nomenclature conventions and steer clear of the more obtuse pharmaceutical-like derivations.
GO TO:
| Home | Overview | People | Journal | Nutrition |
| Environment |
|
WWW Links | Outline | e-mail us |