Copyright © 2000, 2001 by Galen
Daryl Knight and VitaleTherapeutics, Inc.
2-D and Other NMR of Beta-Alethine and Vitalethine
Dr. Jan Dahmen, at the time with Astra Draco, has done
some of the more definitive NMR studies on samples of his beta-alethine
(dihydrochloride) and upon authentic samples of vitalethine supplied by
Dr. Knight. The first step in working out the chemistry of these two compounds
with NMR is to decide which carbons and which proton peaks in the NMR spectra
are associated with each methylene group.
Using 2-D NMR, Dr. Dahmen accomplished this by looking
first at long range coupling effects of protons and carbons to determine
which methylene was farthest removed from the carbonyl (C=O) of the amide
in the center of the beta-alethine monomer:
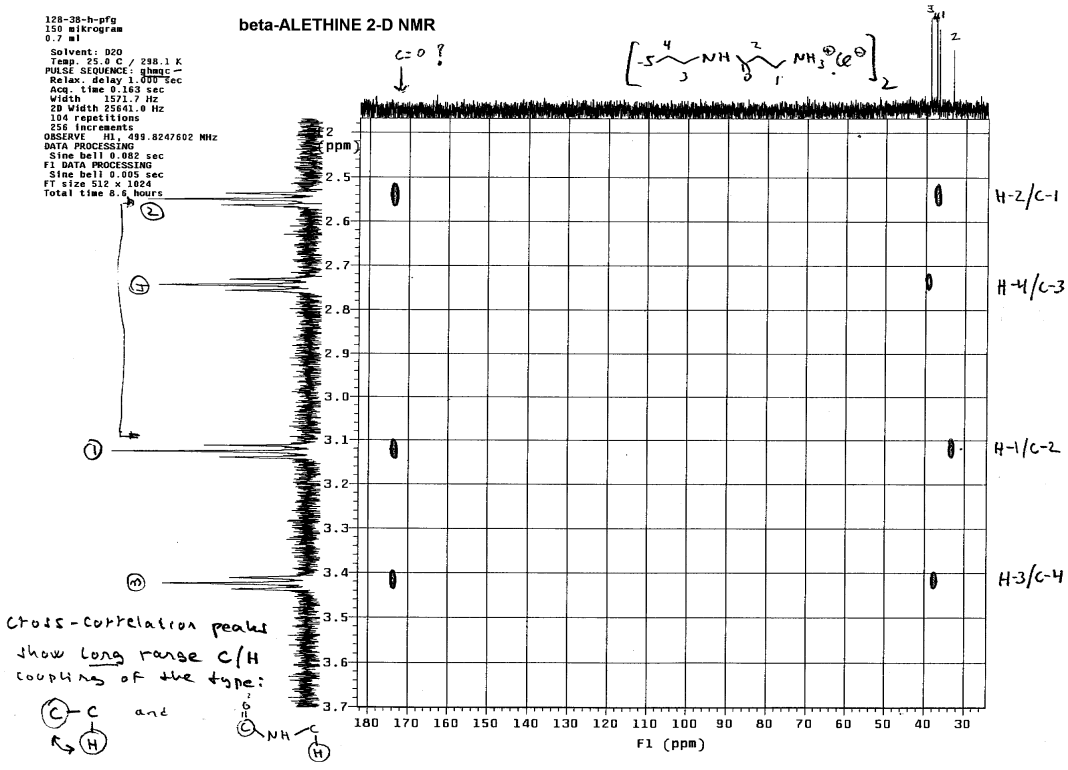
Only the methylene (4) whose proton NMR peak is between 2.7 and 2.8
lacked long range coupling with the carbonyl carbon. Coupling constants
between methylenes that are chemically bound to one another are usually
very similar, so one can determine which methylene is bound to #4 by comparing
the coupling constants. This methylene is of course the #3 methylene, which
was confirmed by Dr. Dahmen using T1 measurements: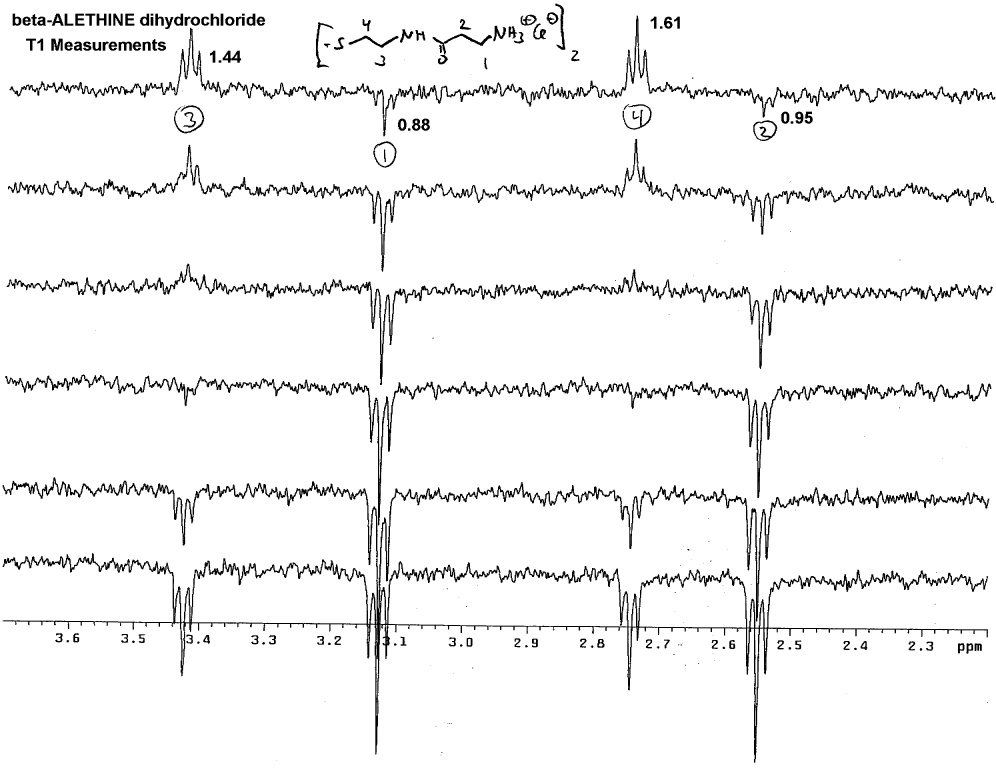
Note that # 3 and #4 methylenes always wind up on the same side of
the spectra, whereas peaks for methylenes #1 and #2 can wind up on the
opposite side of the spectra pointed away from the peaks for #3 and #4.
By default, this helps to identify which peaks are #1 and #2 and these
can be distinguished from one another using the additivity rule to calculate
which peak is which. Methylenes that are adjacent to carbonyls (C=O) typically
are shifted downfield less than methylenes adjacent to amines which have
higher ppm downfield shifts. Dr. Dahmen confirmed this by looking at short
range 2-D couplings of proton and carbon spectra: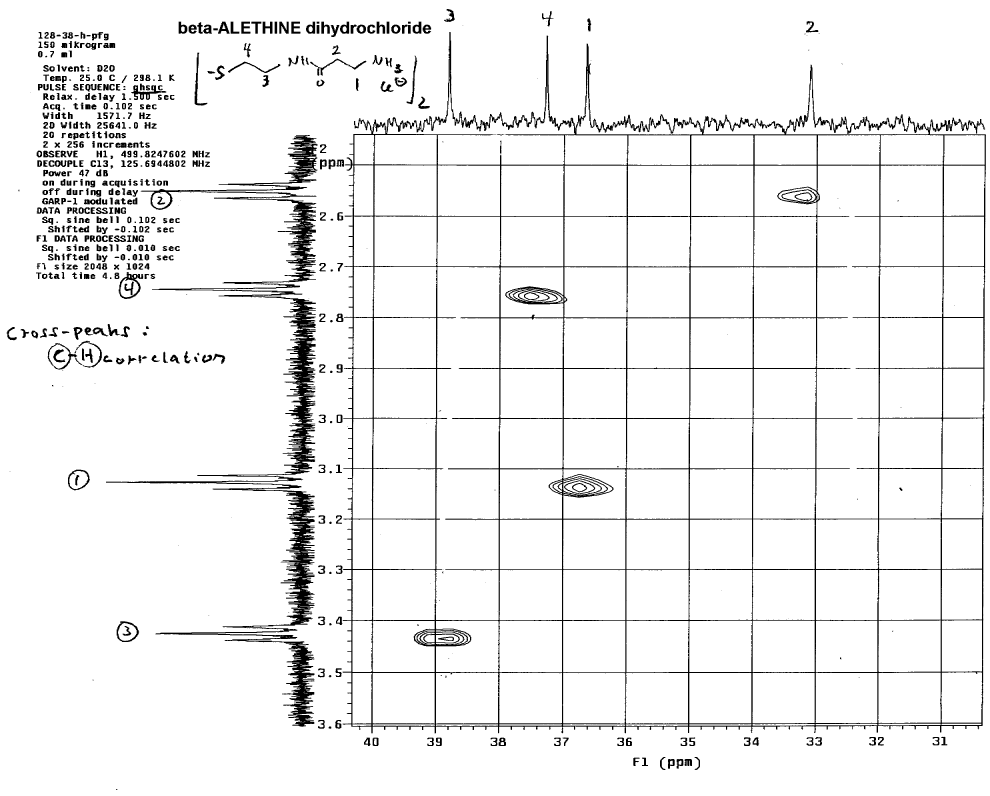
This technique helps to identify which carbon in the carbon NMR spectra
is associated with each set of protons in the proton NMR spectra.
Dr. Dahmen's comparison of the proton NMR of these
two compounds show what appears to be some real differences: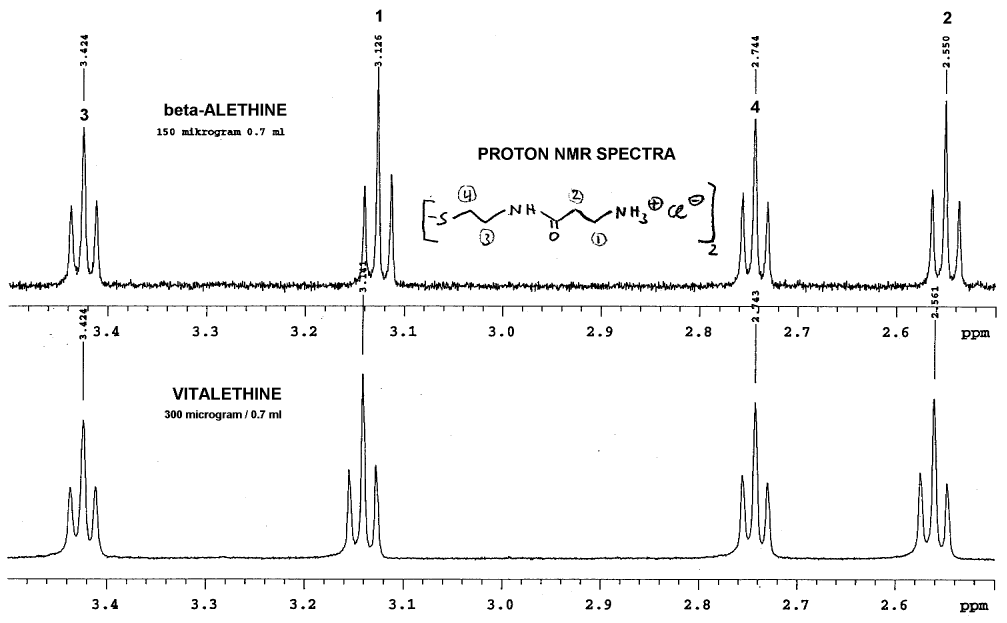
Note that there was no lock signal for these spectra, so some of the
peaks have been aligned for identification of the peaks that do NOT align.
This alignment is probably not far off, since the methylenes in positions
1 and 2 are shifted downfield as expected for vitalethine
when compared carefully with beta-alethine. Since the vitalethine prepared
by Dr. Knight was neutralized with ZnO and Dr. Dahmen's beta-alethine was
not, one can NOT automatically assume that Dr. Dahmen's work provides evidence
for the carbamic or carbonimidic tautomer of vitalethine, even though his
observed shifts in positions one and two are consistent with the downfield
shifts expected for a carbamic or carbonimidic tautomer relative to the
free amine based upon additivity calculations. Fortunately, in the comparisons
performed by Drs. Knight and Morrow, beta-alethine and vitalethine were
both neutralized with ZnO and the signal was locked to water as a standard.
Thus, the data from Dr. Dahmen is consistent with the downfield shifts
for vitalethine relative to beta-alethine observed by Drs. Knight and Morrow,
and consistent with a real chemical difference between these two compounds.
Dr. Dahmen's analysis of these two compounds with
carbon NMR also produced some additional interesting results: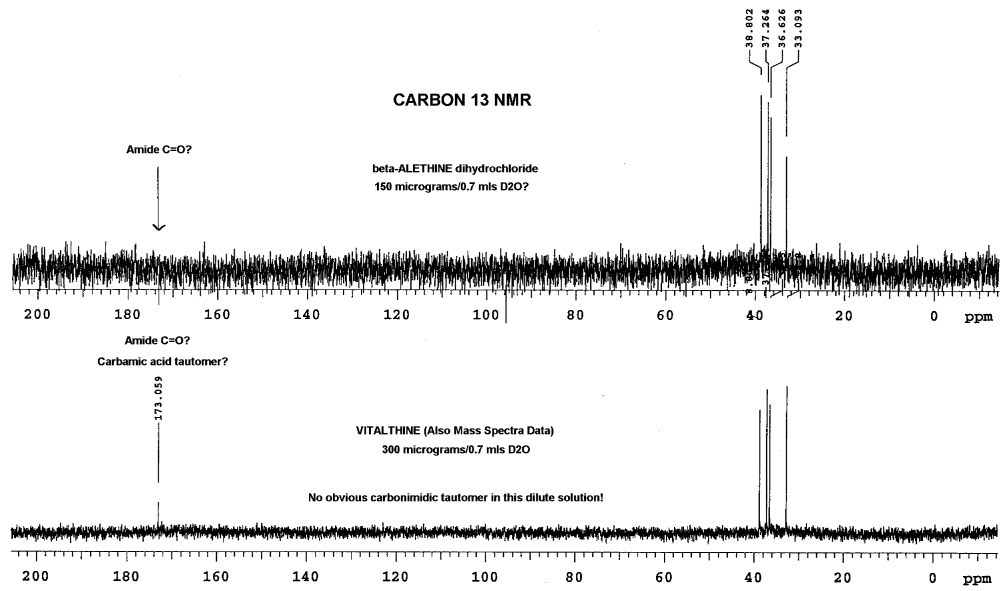
In this analysis, Dr. Dahmen did not observe the carbonyl peaks of
the amides in beta-alethine, whereas, a peak is clearly visible in the
vitalethine preparation at 173. While it is tempting to speculate that
this is the carbamic acid peak for vitalethine, one not expected to be
found for beta-alethine, one must be wary of the differences in the relative
amounts of these two compounds used in obtaining these spectra. Vitalethine
was twice as concentrated, so the peak observed may very well be the amide
carbonyls (C=O), instead of the carbamic acid peak. This difficulty in
seeing peaks that aren't enhanced with NOE, such as beta-alethine's amide
carbonyl peak (C=O) in the upper spectra, illustrate the importance of
using concentrated samples for analysis, especially in carbon NMR determinations.
Drs. Knight and Morrow had more vitalethine (100
mg) and beta-alethine available to do a comparison
of the carbon spectra of these two compounds than Dr. Dahmen, explaining
the pronounced carbonyl (C=O) peaks and the appearance of the carbonimidic
peak in their carbon NMR spectra of authentic vitalethine. Though suspect,
it is not known at this time if the concentration of vitalethine in solution
influences the equilibrium between the carbamic and carbonimidic tautomers.
Blowing up Dr. Dahmen's carbon NMR spectra, and
aligning as before, again without the benefit of a lock signal, shows differences
in the carbon NMR between the beta-alethine and vitalethine:
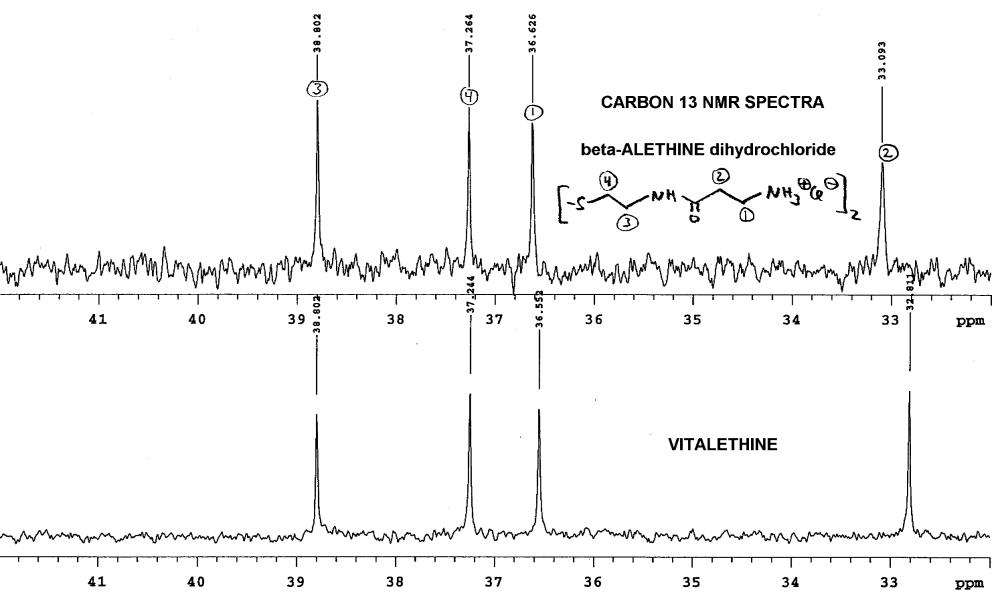
Note that perfect alignment is, again, not possible. Without carefully
controlling pH and concentration, one cannot definitively rule out physical
factors as the cause of these spectral differences, but Dr. Dahmen's results
are consistent with other real chemical and biological differences observed
between beta-alethine and vitalethine. In this case, the methlene most
likely to be affected by a carbonate ester of the terminal amine of beta-alethine
is either the terminal methylene (1) or the methylene (2) adjacent to the
carbonyl of the amide. By pulling the amide moiety into an imidate tautomer,
hydrogen bonding of the carbamic acid moiety with the central amide could
cause steric constraints and changes in the shielding of the methylene
adjacent. Any increased shielding on the imidate carbon would be expected
to move the chemical shift of this carbon upfield, as observed.
It is interesting that the assumption by Dr. Knight,
that the downfield peaks in the beta-alethine
and vitalethine comparison are both amide carbonyls (C=O) with similar
spectral shifts, in his alignment of the carbon NMR spectra of these two
compounds may be incorrect. The peak shifted downfield (high ppm) in the
vitalethine preparation could be some combination of carbonyl peaks from
both the amide and the carbamic acid tautomer. Furthermore, little is known
about the chemical shifts for imidate tautomers or about the effects of
hydrogen bonding upon chemical shifts in the proton and carbon NMR spectra
for this family of compounds. More, careful research is obviously needed.
GO TO:





