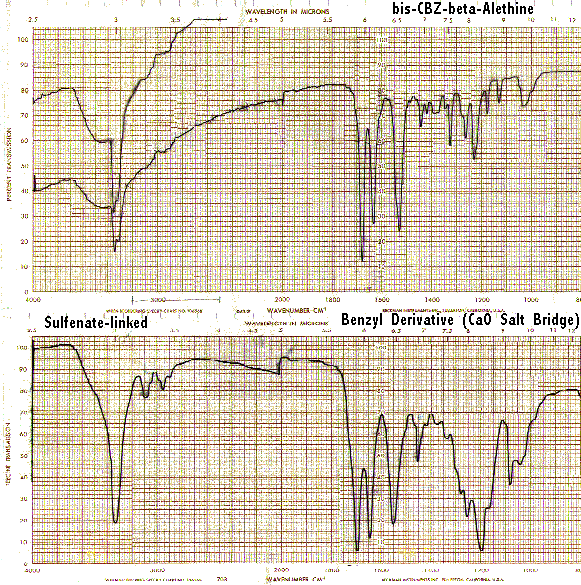

It is interesting to note that ß-alethine when neutralized displays little of the band at 1560/CM characteristic of vitalethine when it is similarly neutralized with ZnO.
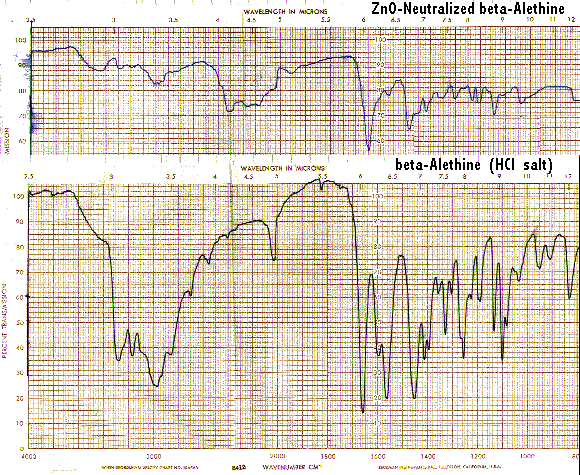
These IR spectral differences between ß-alethine and vitalethine disappear when each are heated to their respective melting/decomposition points.
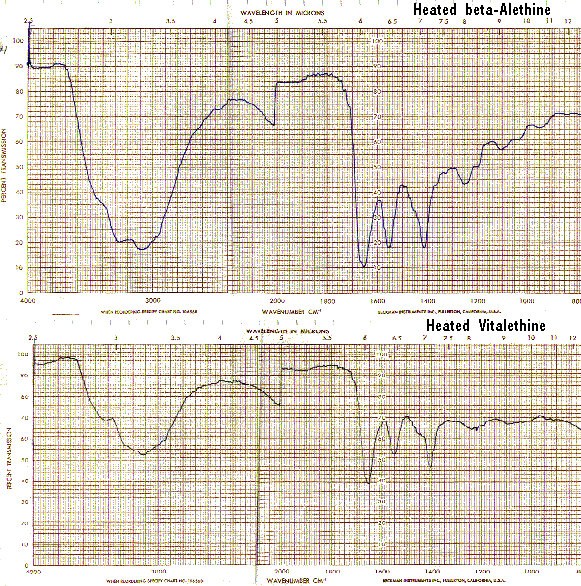
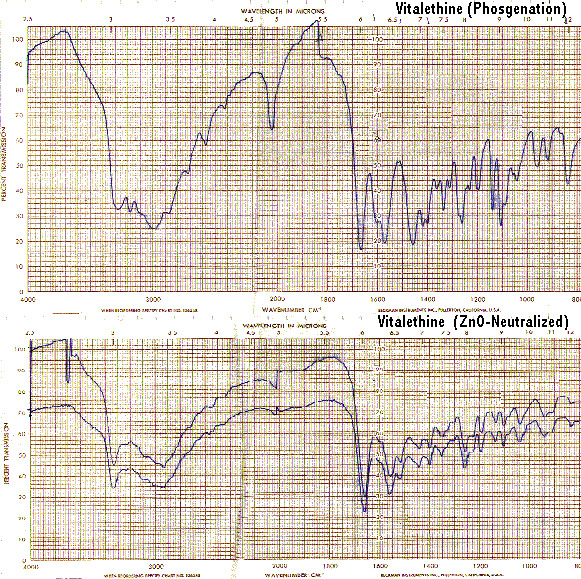
Bands in the carbamate/carbonimidate regions also disappear when vitaletheine V4 is heated to its decomposition point, corroborating the structural assignments for both vitalethine and vitaletheine V4.
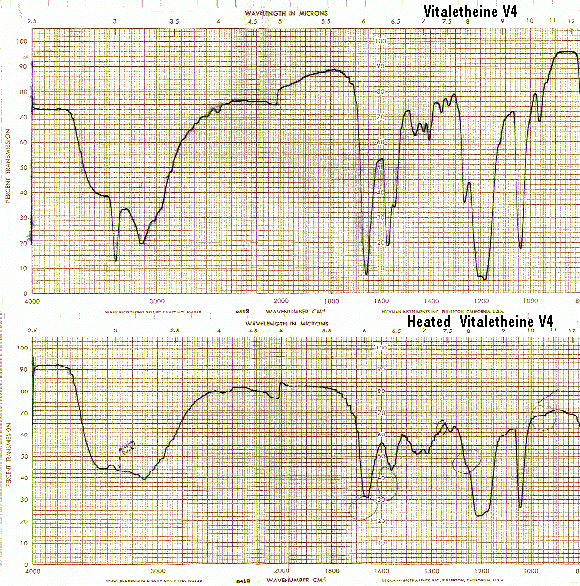
The IR spectra of heated vitaletheine V4 also resembles that of ß-alethine (supra), but the decomposition product of the C-O-linked tetramer has strong bands in the C<->O/C<->O-C region(1310-1000/CM). This indicates that the ring structure of vitaletheine V4 may be more stable than expected, possibly due to 5-and 6-membered hydrogen bonding within the structure. As noted before in relation to the sulfenate-linked benzyl derivative these C<->O bands are for the most part absent from bis-CBZ-ß-alethine. The spectra of heated sulfenate-linked benzyl derivative also has some of this same C<->O character, but it is somewhat less pronounced than in vitaletheine V4, indicating differences in the C<->O moieties responsible for these peaks and possibly less conversion to the tetramethylenoxy-linked thermal decomposition product.
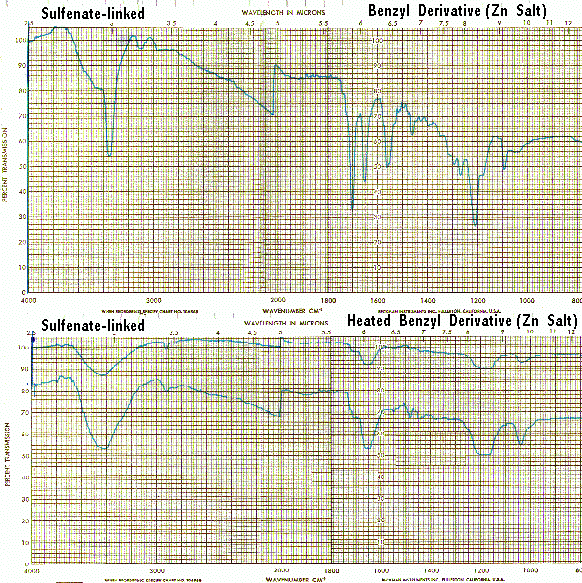
When heated, the sulfenate-linked benzyl derivative also does not display the band at 1560/CM typical of other heated spectra (supra). This suggests that the attachment of monomers in the sulfenate-linked benzyl derivative and in vitaletheine V4 differ. Also, as noted previously, the loss of similar bands from the spectra of both vitalethine and vitaletheine V4 provide corroborating evidence for the carbamate/carbonimidate structures in these two compounds.
The single strong band in the C<->O region for authentic samples of the sulfenate-linked benzyl derivative apparently have been misinterpreted as superimposed S<=>O bands (1250-1140, 1070-1030/CM) by others. However, the far IR spectra of authentic compounds provides additional evidence that the authentic benzyl derivative and vitaletheine V4 are not sulfonates, nor even sulfinates for that matter. Sulfinate and sulfonate derivatives are expected to have more strong bands in this region than the spectra of either the authentic benzyl derivative (a sulfenate-linked dimer with C<->O bands) or vitaletheine V4 (a thiol with nearly equivalent C<->O bands). Printing in landscape mode will allow capture of the full spectra, following:
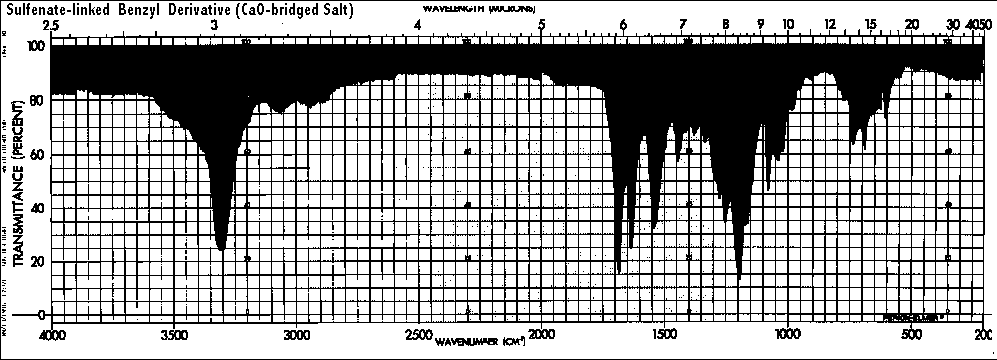
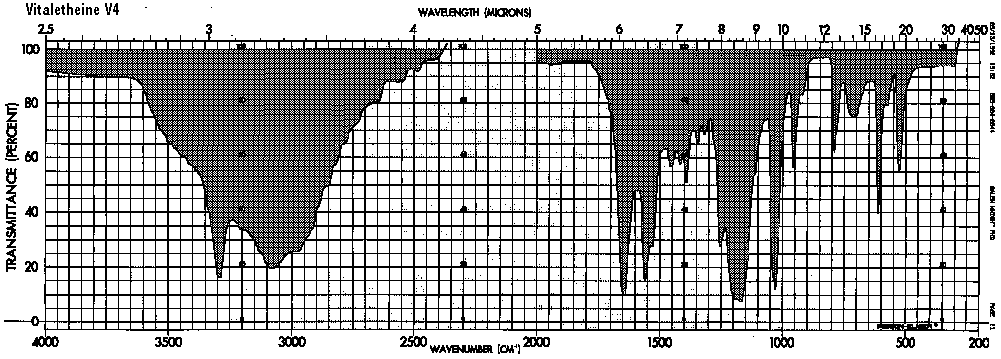
Also, if both of the authentic compounds were sulfonates, there should be similar bands for the sulfonates or sulfonic acids below 700/CM in both cases. The bands in the sulfonate-linked benzyl derivative are at 740, 690, and 610 which are more consistent with S<->O (870-690/CM) and perhaps carbamate/carbonimidate moieties than with sulfonate absorbances. Also, the C<->O<->C at about 955/CM for the authentic vitaletheine V4 (1050-900/CM) is too low for the medium intensity bands expected from sulfonates, and this band, as expected from the structural assignments, is absent altogether from the spectra of the authentic, sulfenate-linked benzyl derivative and ß-alethine.
Based upon other accumulated NMR and elemental analyses, the carbonimidate, thiol, and disulfide moieties probably explain the bands at 790, 710, 610, and 525/CM) in vitaletheine V4 and even may indicate some autoxidation of the thiols in the tetramer at about 600 to disulfides at 525 in the above preparation. As expected for its stable sulfenate-linked structure, there is no IR evidence for decompositions or autooxidations of authentic benzyl derivative (zinc salt) after nearly three years of storage (refrigerated and frozen under ambient conditions).
Comparing the peaks in the sulfenate-linked benzyl derivative with covalent carbonates and imino carbonates, the bands for the carbamate and presumably the carbonimidates appear at about a 150/CM lower wavenumber, probably accounting for the two bands in the 800-700/CM range of vitaletheine V4; there are no sulfonate peaks associated with this region of the spectra. Coupled with the observation that the C<->O-C band in vitaletheine V4 (1050-900) is much more pronounced than in the sulfenate-linked benzyl derivative, there is every reason to believe that authentic vitaletheine V4 is the indicated tetramethylenoxy-linked polymer.
Other rationale supports this distinction. If both the benzyl derivative and vitaletheine V4 were sulfonates, logically, they would have more in common in the C-O and C-O-C regions of the spectra; the sulfur moieties are far-removed from the attachment site for the carbobenzoxyl blocking group of the sulfenate-linked benzyl derivative and its spectral influences. Finally, if both the benzyl derivative and vitaletheine V4 had an amide backbone identical with ß-alethine, the situation necessary to invoke the sulfonate assignments, one would be hard-pressed to account for the striking differences observed between the full IR spectra of this base structure and each of these two authentic vitaletheine modulators, vitaletheine V4 and the sulfenate-linked benzyl derivative. This indicates that the sites of attachment for the monomers in these established polymers involve the amides, thereby accounting for the observed differences in the IR spectra and confirming the structures of the authentic sulfenate-linked benzyl derivative and tetramethylenoxy-linked polymer, vitaletheine V4.
GO TO:
| Home | Overview | People | Journal | Nutrition |
| Environment |
|
WWW Links | Outline | e-mail us |