Copyright © 1997, 2001 by Galen Daryl Knight
and VitaleTherapeutics, Inc.
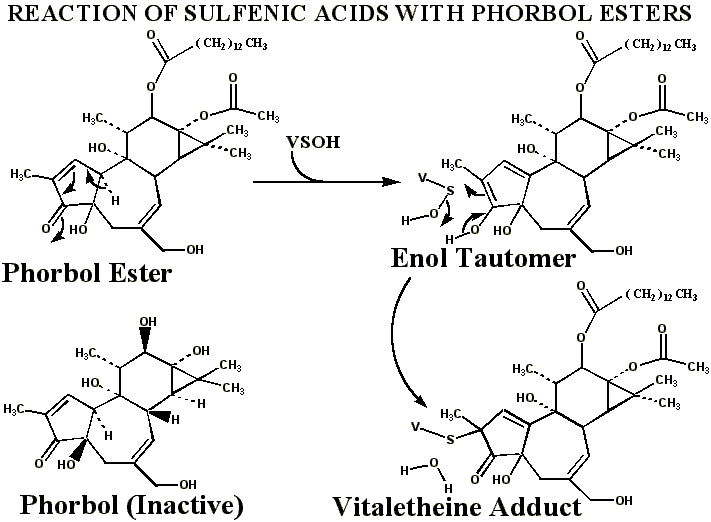
Alkylation of Vitaletheine's Sulfenic Acid with Phorbol Esters
Although
other possible explanations exist
for the cancer promoting activities of phorbol esters, the ester groups
of these compounds are recognized as being essential for their undesirable
activities. The long hydrocarbon chain esterified to phorbol may effectively
anchor the otherwise water-soluble,
phorbol
moiety in the membrane where the monooxygenase is located and where
this enzyme is generating sulfenic acids of the vitaletheine modulators;
without the ester moieties, phorbol is far less potent as a tumor promoter.
Tautomerization to the enol in this phorbol portion provides an effective
reagent for removing sulfenic acids of the vitaletheine modulators. This
tautomerization probably is not direct, but can occur through a conjugation
within the 5-membered ring containing the ketone. The enol tautomer, as
in many other toxins, is probably stabilized by the hydroxyl group vicinal
to the keto group.
GO TO:
