VITALETHINE AND BETA-ALETHINE:
AUTHENTIC MASS SPECTRA vs. ARTIFACTS
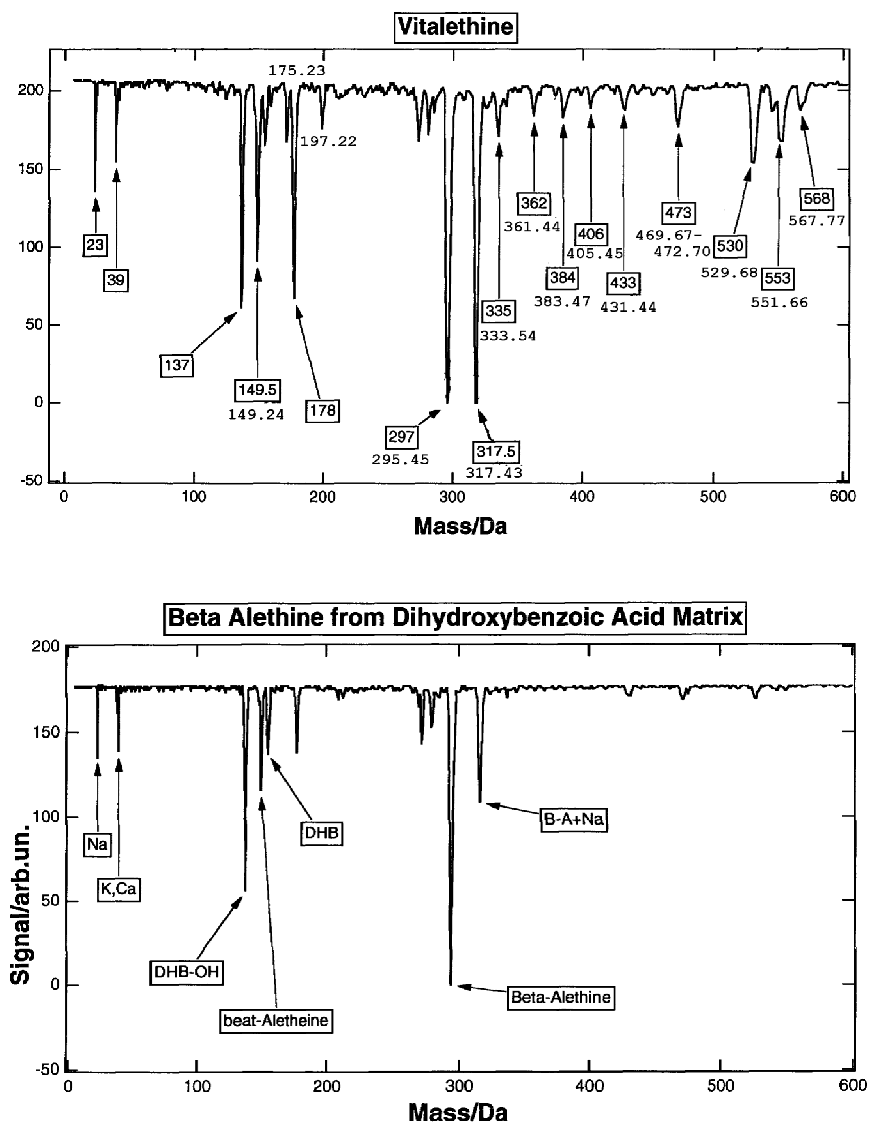
The mass spectra of authentic vitalethine produces slightly
higher mass ions [in boxes] than expected (under boxes), probably
because it was taken from deuterated water and some of its hydrogens
(on the nitrogens and on the carbamic/carbonimidic portions of the
molecule) probably exchanged with the [heavier deuterium]. The mass
ion, for example for vitalethine is
observed at 384 and 406 (Na+ adduct)
instead
of 383.466 and 405.448, the small peaks for masses also being broad
instead
of sharp because of these isotopic effects.

The preparations contaminated with the brominated, bis-acetone
Schiff bases, and with mixed disulfides with cysteamine, present
issues of authenticity, stability, and safety. These contaminated
preparations are reportedly tens of thousands of times less potent than
authentic beta-alethine in biological assays, possibly confirming
suspicions that the biological activity of authentic beta-alethine
results from traces of vitalethine (masses observed at 433, 473, 530)
formed by equilibration of beta-alethine with atmospheric carbon
dioxide, just as carbamino adducts are known to form with the amino
groups in hemoglobulin (respiration) and in the protein in plants
involved in trapping CO2 for photosynthesis. The bis-acetone
Schiff bases on beta-alethine artifactually prevent this CO2/carbamate
equilibration from occurring, and because of the structural similarity
of this artifact
to vitalethine, these contaminants probably also competitively inhibit
the
biological activities of vitalethine. Hence, the dramatic drop in
potency
and stability of beta-alethine when others fail to completely removing
bromide ions and through their failure to use the right solvent, both
failures being devastating departures from the authentic synthetic
procedure that uses acetonitrile instead of the problematic acetone.
Logically, then, the Schiff bases with contaminating acetone preclude the formation of most (if not all) stable vitalethine by blocking the free amino groups of beta-alethine needed to react with carbon dioxide or with phosgene to form vitalethine, through natural equilibration or in the authentic synthetic procedure, respectively. The contaminating bromide ions also tend to prevent the formation of vitalethine by helping to disproportionate the desirable disulfide into the thiol or reduced form of vitalethine, the thiol form being very difficult to synthesize, chemically, probably because of the tendency of the thiol to attack the amide intramolecularly, and the tendency of the resulting amino-ortho ester to then attack the carbamic/carbonimidic ends of vitalethine, thereby altering the terminal carbamic/carbonimidic functional group that makes vitalethine about a trillion times more potent than beta-alethine.
Note that the polymerized or higher aggregate masses observed in vitalethine, only traces of which were also found in beta-alethine, only appear in the MALDI mass spectrometer, and that these probably result from polymerization, or at least complexation reactions caused by the UV laser in the mass spectrometer. These reactions are no doubt similar to 1) those UV-polymerizing reactions already proposed for these types of compounds in the ortho-ester-like synthesis of authentic vitaletheine V4 (the tetramer), and to 2) the ortho-ester-like, highly therapeutic sulfenate-linked benzyl carbamate dimer. The proton and carbon NMR for the authentic preparations of beta-alethine and for authentic vitalethine confirm this conclusion, since these authentic preparations appear to be pure, with little (if any) NMR evidence for the heterogeneity and decompositions observed in the MALDI mass spectrometer.
GO TO:
| Home | Overview | People | Journal | Nutrition |
| Environment |
|
WWW Links | Outline | e-mail us |