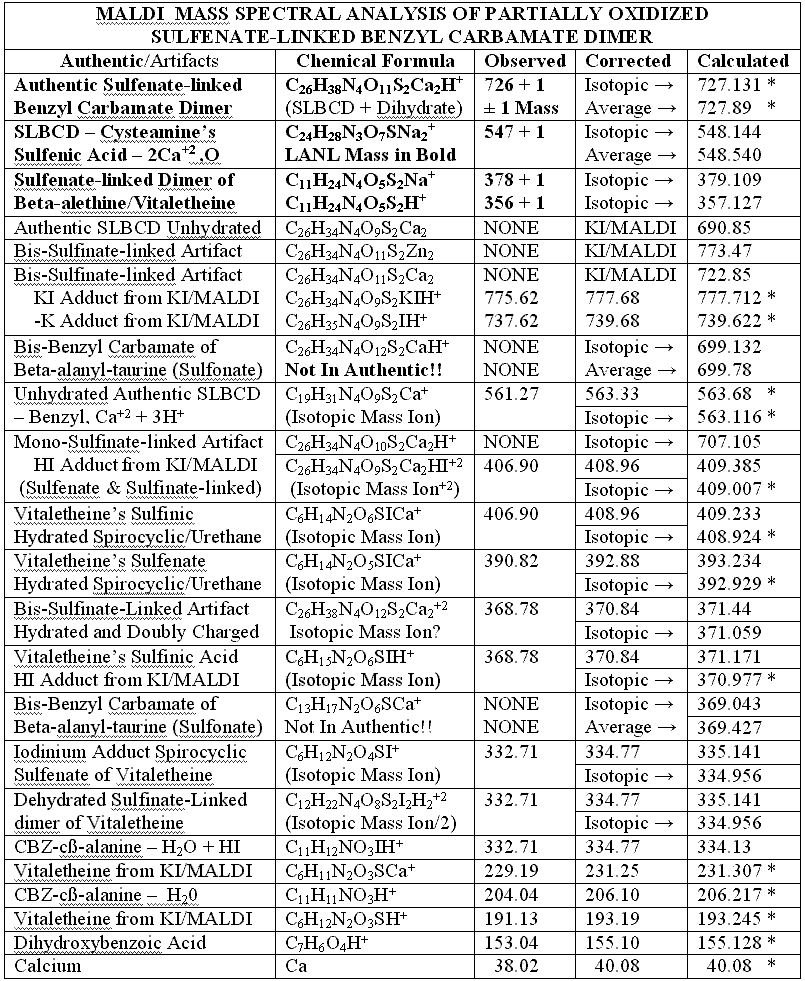
MASS SPECTRAL STRUCTURAL PROOF? JUDGE FOR YOURSELF!
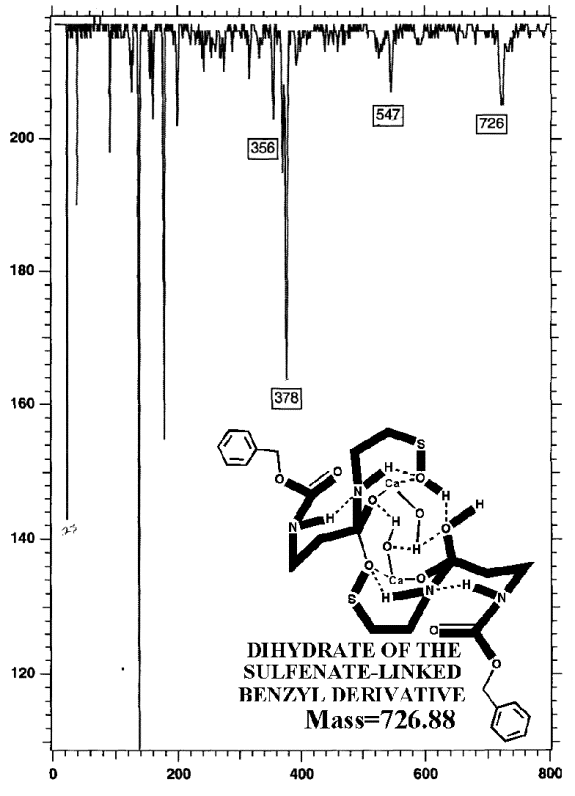
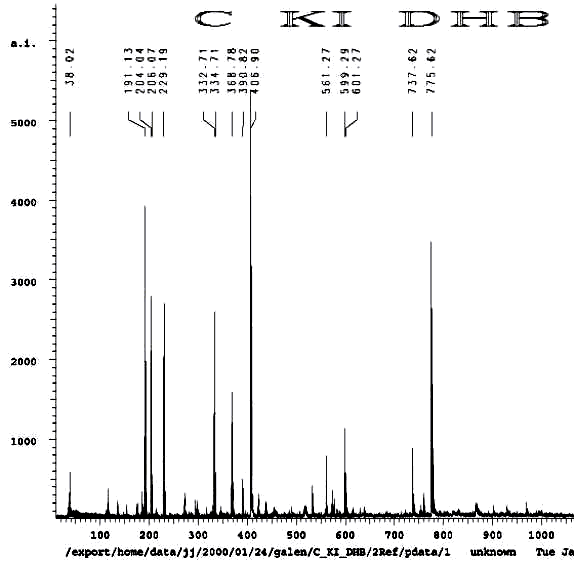
POTASSIUM IODIDE AS SHIFT REAGENT
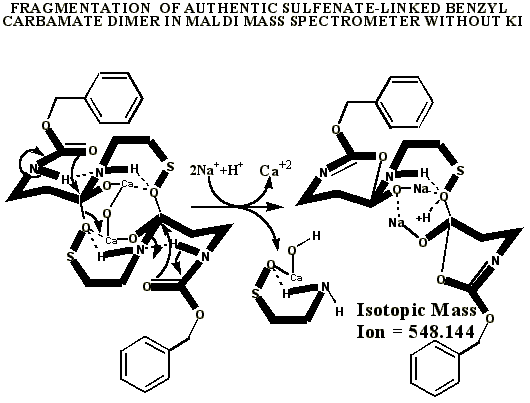
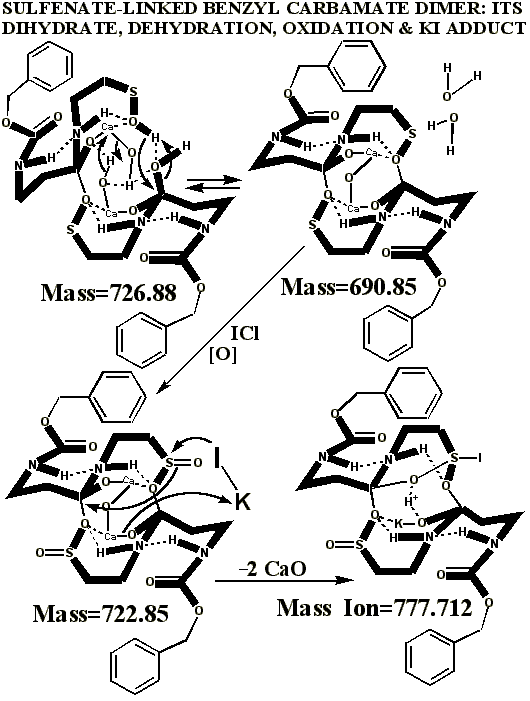
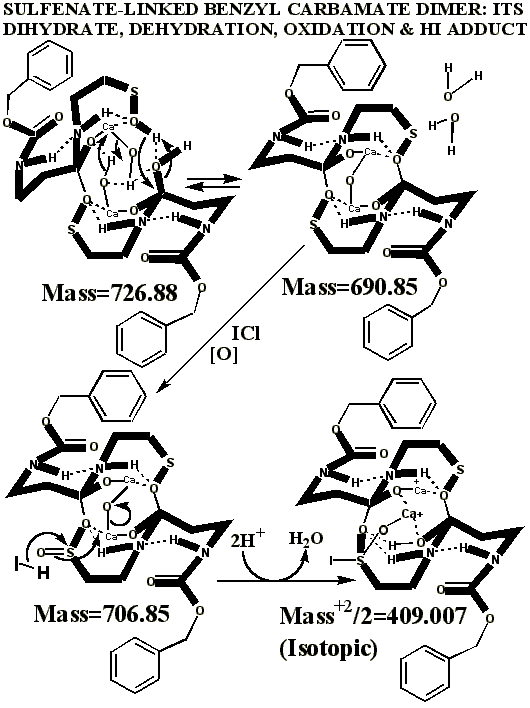
KI's presence in the MALDI analyses also causes reduction of sulfenates and sulfenate-linked compounds to thiols. It has long been known that sulfenates react with iodide to form sulfenyl iodides , which are then reduced to thiol compounds by ultraviolet light, such as that emitted by the UV laser of the MALDI mass spectrometer. (Thiols and unreacted sulfenates or sulfenyl iodides form disulfides.) Thus, sulfenates and sulfenate-linked compounds tend to be reduced by iodide to smaller sulfhydryl products in the MALDI mass spectrometer, whereas sulfinates and sulfinate-linked compounds tend to form large complexes with iodide that apparently do NOT reduce into the smaller thiol compounds. A second signature peak for the artifactually oxidized authentic compound is observed at 739.622 when KI is present under these acidic conditions:
Presumably, sulfonates and sulfonate-linked compounds, due to their high oxidation states, are even more resistant to reduction to thiols under these conditions, and may even be resistant to adduct formation with iodide. This last suspicion remains to be tested, since there is absolutely no evidence for sulfonates or sulfonate-linked compounds in the authentic preparations, even when the authentic synthetic process using iodine was contaminated with HCl (ICl) to control a pH overshoot. It is highly unlikely that iodide and UV light could completely reduce a sulfonate to a thiol in the split second between its desorption with a UV laser and its analysis by the detector of the mass spectrometer, especially when even the sulfinate-linked, -S(O)-O-, compounds appear resistant to such reduction. Full reduction of the sulfonic acid to sulfide or thiol would require the highly unlikely chance meeting of the sulfonic acid with several iodide ions in the UV-illuminated flight path of the mass spectrometer.
Having chloride ions from HCl present in the iodine reaction constitutes a serious departure from the authentic procedure, since the iodine monochloride ( ICl ) so generated is known to be extremely reactive, and capable of artifactually oxidizing sulfur compounds to higher oxidation states. Similar polarity problems exist for reactive mixtures of bromine and chloride .
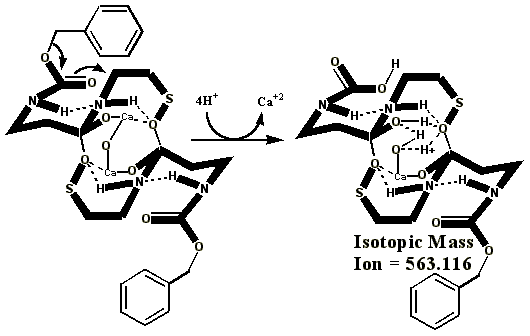
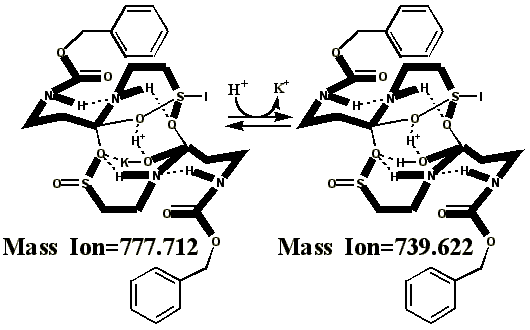
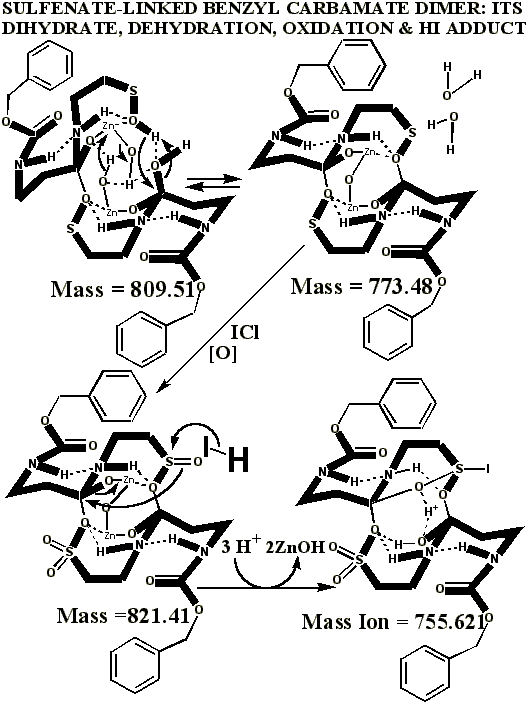
Additional proof that the authentic sulfenate-linked benzyl carbamate dimer, -S-O-, is not the bis-sulfinate-linked structure, -S(O)-O-, is provided by mass ion adducts that are intermediary between the two, especially in preparations known to be contaminated with HCl. While a mechanism can be drawn for the sulfenate-linkage being reduced by iodide, presumably the sulfinate-linkage is less likely to be reduced to thiol and, therefore, more likely to form a stable adduct with iodide, the doubly charged mass ion of which is observed in the MALDI mass spectrometer when KI is present:
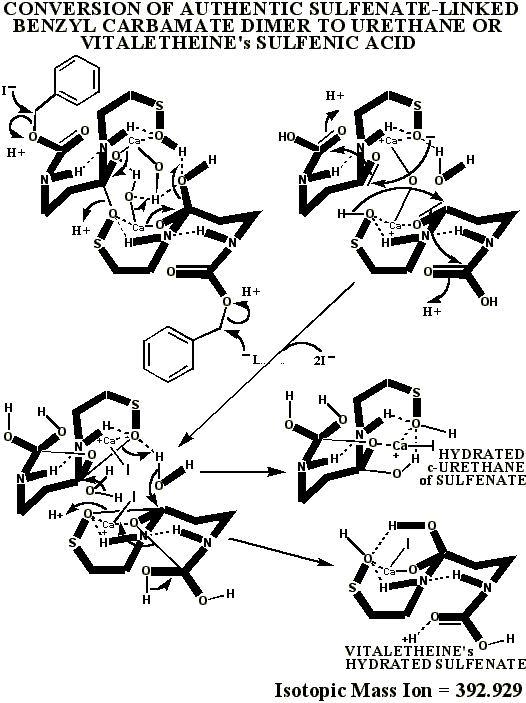
This authentic sulfenate-linked structure is the only one that can account
for the many definitive mass ions observed in the authentic and partially
oxidized preparations. Similar analyses of pristine, pharmaceutical grade
material, prepared by Dr. Knight, is expected to confirm these conclusions,
the oxidative artifacts being expected to drop out of the MALDI mass spectrum
of this pristine material, as the sulfenate and thiol mass ions and adducts
of vitaletheine increase in relative abundance when analyzed in the presence
of KI. The following table summarizes the compelling mass spectral evidence
for the authentic sulfenate-linked benzyl carbamate dimer, and for oxidative
artifacts generated, therefrom, when the otherwise authentic preparation
was contaminated with chloride ions:
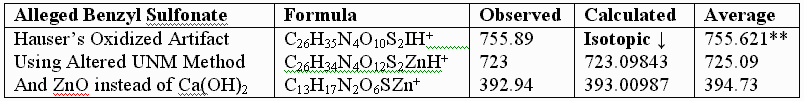
**Attempts by Dr. Murray allegedly using UNM’s method produced an oxidized artifact of the authentic vitaletheine modulator (755.89), but this product from Hauser Chemical clearly lacks the mass spectra fingerprint of the authentic compound. His attempt produced only tiny traces of the 723 mass expected for the alleged sulfonates. The artifactually oxidized authentic preparation, contaminated with HCl and discussed previously, apparently did not fully reduce, and instead produced some large, artifactually oxidized adducts with iodide. Similar resistance to KI/UV reduction is expected for the sulfonates alleged by Drs. Wallace and Murray. In contrast, the unoxidized SLBCD remaining in the partially oxidized preparation appears to be readily reduced by KI and the MALDI's UV laser to vitaletheine and other small fragments and adducts.
One possible explanation for Dr. Murray’s artifact, produced through his admitted departures from the authentic procedures, including much longer reaction times with iodine, is the much higher oxidation state at 755.621 than observed for the authentic compound. The calculated value, following, is in good agreement with Dr. Murray’s observed mass ion adduct at 755.89, the 723 mass for the alleged sulfonate being much smaller and virtually absent from the mass spectrum of Dr. Murray’s alleged attempt:
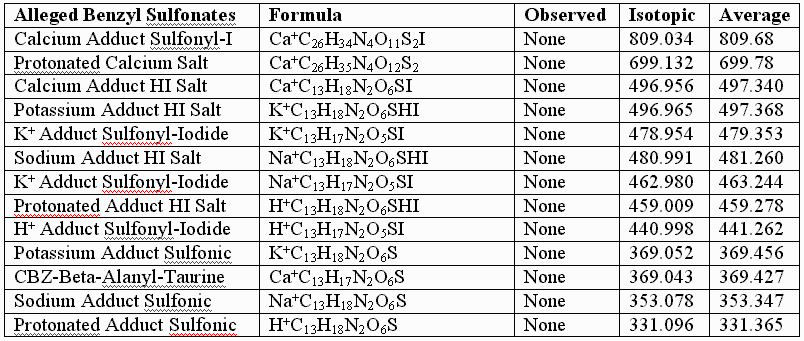
This failure of the alleged sulfonates to fit the observed data for the authentic compounds is further illustrated by calculations performed for the alleged sulfonates, the UV laser of the MALDI instument conceivably capable of removing the benzyl groups from the alleged sulfonates, but NOT capable of reducing the sulfonates to thiols, especially in the absence of iodide. As before, the following table provides few, if any, alternative "sulfonic" assignments that provide better "fits" than those graphically illustrated, supra, for the authentic, and for the somewhat artifactually oxidized preparations:
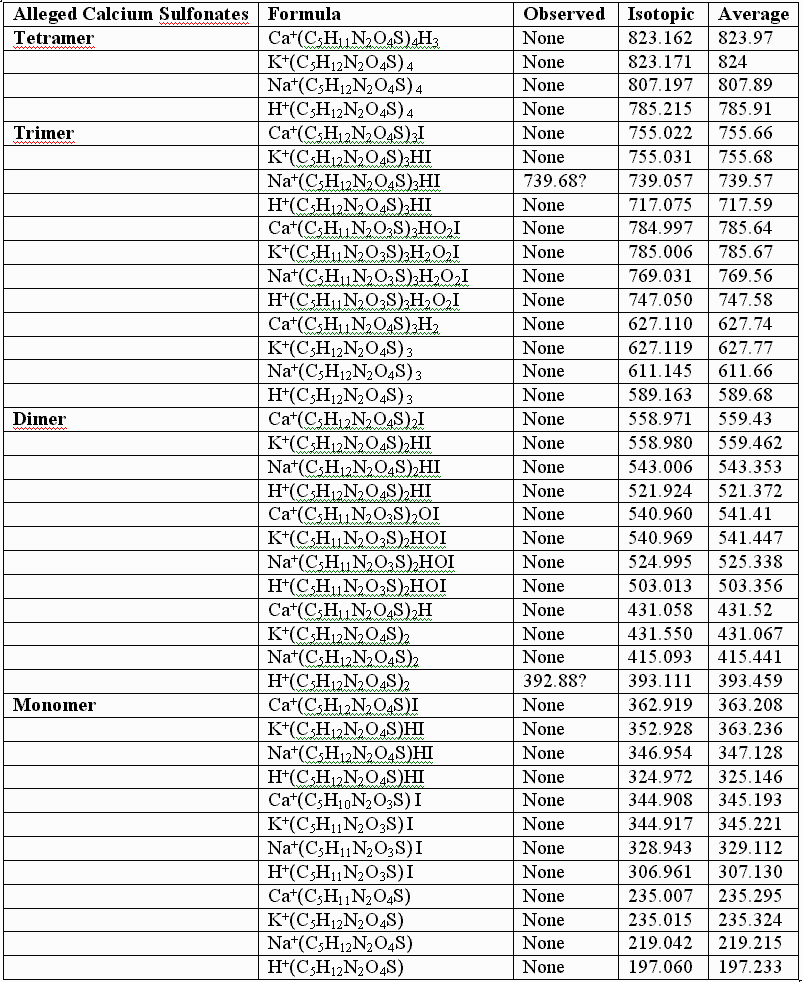
In conclusion, UNM, its licensee, and their so-called "experts" appear to be simply wrong about the structure of the authentic sulfenate-linked benzyl carbamate dimer. The masses assigned in the first table, supra, for the authentic sulfenate-linked benzyl carbamate dimer, and for oxidative artifacts thereof, appear to produce an accurate "mass signature" for the authentic sulfenate-linked compound. This hasn’t stopped UNM and its licensee from promoting the alleged sulfonate or taurine derivatives for human use, as if they were the same as the authentic compounds, and apparently without much testing of these alleged sulfonate and taurine derivatives in animals for biological efficacy and safety.
GO TO:
| Home | Overview | People | Journal | Nutrition |
| Environment |
|
WWW Links | Outline | e-mail us |