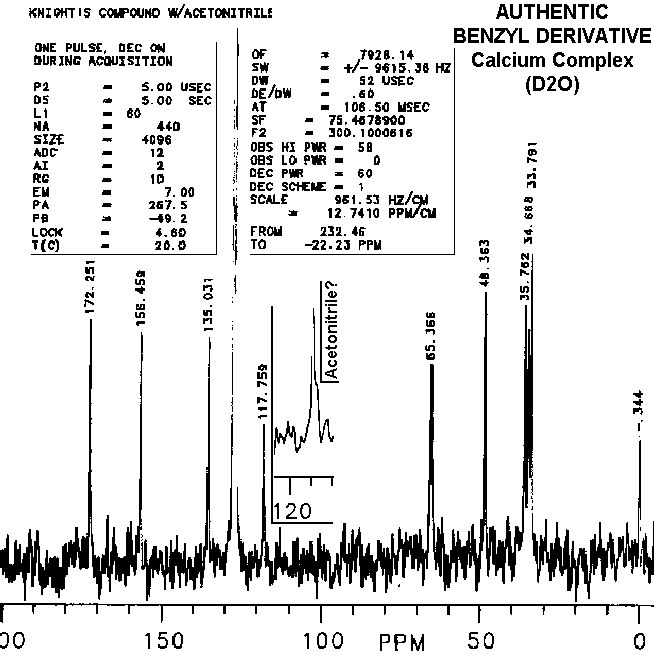
Disappearances of peaks in the 110 to 120 ppm range in other's preparations of the "benzyl derivatives" and "vitaletheine V4" are consistent with the proposed mechanisms for dehydration and loss of ZnO (especially when concomitant relative increases in peaks and shoulders above 170 are observed); further decomposition can lead to a complete loss of the carbonimidate/carbamate peaks from the spectra of vitaletheine V4 through decarboxylation. This is indicated by an associated increase in the amplitude and broadening of the proton NMR peak representing the liberated amine.
The slightest indication of an upfield shoulder (inset) on the peak
at about 117-120 ppm thought to be acetonitrile in authentic preparations
of the benzyl derivative is not well represented after photocopying, scanning,
and translation into gif files. This peak and shoulder are absent from
spectra of others' preparations, indicating both, removal of acetonitrile
and dehydration, in their attempts. Also consistent with a theoretical
dehydration is the observation that the relative amplitudes of
peaks
above 170 ppm are higher in other's preparations of "benzyl derivative"
than in the authentic preparations. Although differences in peak intensities
and relative positions are quite small between the spectra of authentic
compounds and those of the
alleged sulfinates and
sulfonates, these differences are discernible upon close inspection
of the higher resolution spectra.

The technical expertise of Dr. Frank Kroh, now an employee of TPL, Inc., in obtaining the spectra for the benzyl derivative (supra), and of Dr. Cary J. Morrow, then chairman of the chemistry department at UNM, in obtaining the remainder of these carbon NMR spectra, following, is gratefully acknowledged.
The peak at about 120 and other small peaks in the spectra of authentic
vitaletheine V4 would normally be dismissed as solvent and solute contaminants.
However, other chemical data and proton
NMR for this compound are more consistent with the proposed carbamate
structure for vitaletheine V4. For example, the small peak at 40 would
normally be dismissed as contaminating DMSO, but there is very little evidence
for this solvent in the proton spectra of this same sample. Small peaks
in this spectra might represent distributions among the various free ortho-ester,
carbamate, imidocarbamate, and non-degradative
dehydration
and rehydration (solvation) equilibriums.
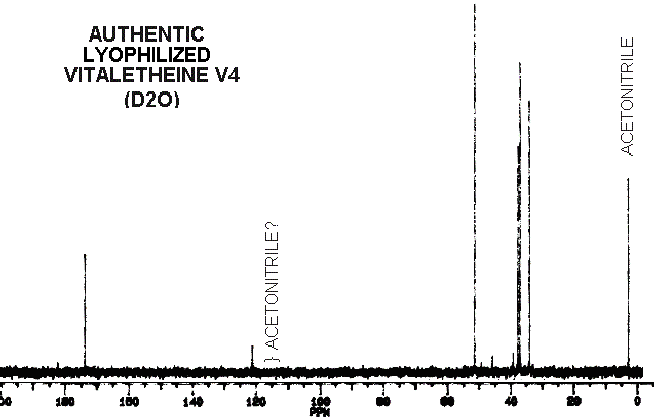
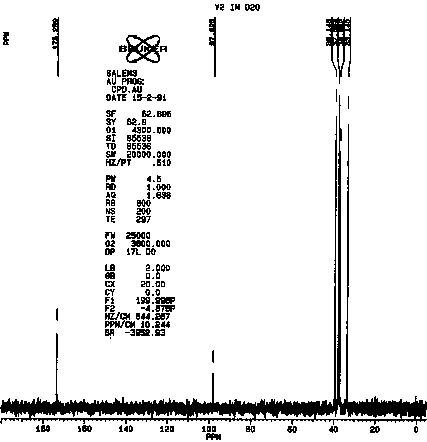
The similarities between vitaletheine V4 and vitalethine are striking,
the only obvious difference being the shift of one of the peaks just below
40 ppm in the spectra of vitalethine to above 50 in the spectra of vitaletheine
V4. This is consistent with polymerization in the former but not the latter,
since the carbon spectra of unpolymerized ß-alethine
is also very similar to vitalethine. Efforts are underway to thoroughly
define the chemistry of the peaks in the 100 to 120 ppm range and their
relationships with tautomers having peaks above 170 ppm. The carbonimidic
tautomer is a likely assignment for the resonance at about 100 in vitalethine
(V2).
GO TO:
| Home | Overview | People | Journal | Nutrition |
| Environment |
|
WWW Links | Outline | e-mail us |