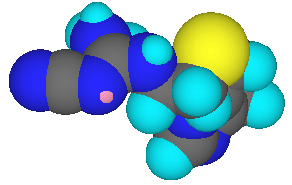
GO TO:
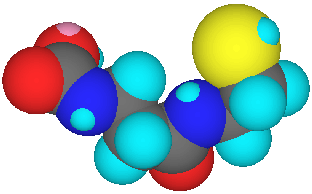
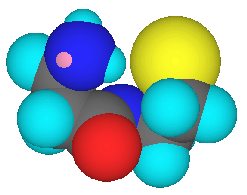
Differences between cimetidine (Tagamet ® SmithKline Beecham) and vitaletheine are numerous, but the cysteamine moiety of vitaletheine (below left) is present in cimetidine as an alkyl sulfide (below center) and the nucleophilic nitrogens (dark blue) of cimetidine are positioned at locations similar to those nucleophilic nitrogens and oxygens (red) of vitaletheine. Cimetidine also bears some resemblance to ß-alethine (below right) suggesting anti-histamine (antacid) potential for the vitaletheine modulators and related compounds.
Despite structural differences, Cimetidine and a related H-2 receptor antagonist, ranitidine (Zantac® Glaxo, Inc.), are still substrates for the FAD-containing monooxygenase, but slow turnovers (low Vmax) on the enzyme make undesirable inhibition of other monooxygenase-catalyzed reactions by these H-2 blockers likely. This concern is justified further by the observation that immunological responses to cimetidine appear to be mediated through suppressor and helper T-lymphocytes and natural killer cells which differs from TH-2 type of immune modulation produced by the vitaletheine modulators.
In contrast to vitalethine's efficacy in treating cancer it is also
interesting that many of the H-1 type anti-histamines
now on the market, like substrates for the monooxygenase
bearing little resemblance to vitaletheine, increase the growth of
tumors in rodents. The observed uncoupling of the
down-regulation of HMG-CoA reductase by n-octylamine, an amine approximately
the same size as histamine, may provide a mechanism for tumor stimulation
by these types of compounds.


GO TO:
| Home | Overview | People | Journal | Nutrition |
| Environment |
|
WWW Links | Outline | e-mail us |