Copyright © 1996, 1997,
2001 by Galen Daryl Knight and VitaleTherapeutics, Inc.
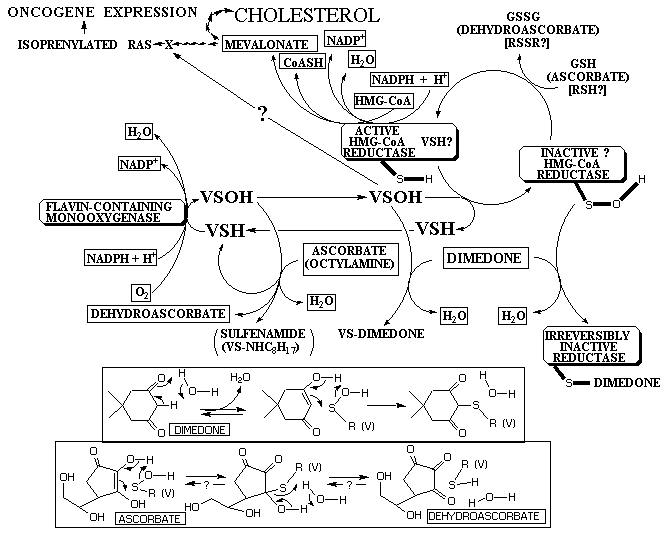
Monooxygenase Control of HMG-CoA Reductase and Oncogenic Expression
The relationship between monooxygenases and HMG-CoA reductase is important
for several reasons. First of all, monooxygenases that catalyze the oxidations
of thiols have the potential for controlling cancer which is dependent
upon isoprenylation of ras, both at the reductase step by blocking the
production of isoprenyl (mevalonate) units and later in the actual isoprenylation
step by modifying (oxidizing) the cysteine residues of ras to preclude
isoprenylation. By decreasing HMG-CoA reductase activity and the production
of mevalonate, monooxygenase activity also can control the biosynthesis
of cholesterol from mevalonate, a contributing factor in heart disease.
The importance of sulfur metabolism in controlling cholesterol is illustrated
by the observed
decrease in dietary cholesterol,
increase in HDL (the good cholesterol), and decrease in triglycerides
in rats fed diets enriched in either cysteine or cystine, as either the
amino acids or protein. Since cysteine is not a substrate for the monooxygenase,
in order for cysteine to work in the following pathway, it must first be
decarboxylated to
metabolites on the Coenzyme
A through cysteamine pathway, which should include members of the vitaletheine
modulator family. The vitamin, pantothenic acid, is required for this to
happen.
Individuals suffering from cystinosis, or some types of kidney stones,
probably should not supplement their diets with cystine or cysteine.
There are some indications in the literature that pantothenic acid supplements
alone may benefit this health problem by helping to metabolize the accumulating
cysteine and cystine to cystamine. If cystinosis is not a problem, then
supplements of either cysteine or cystine can be considered. Cystine may
be safer in the sense that it probably does not extract heavy metals from
any stainless steel processing equipment (used in the preparation of the
supplement) as readily as the more common cysteine supplement (especially
the HCl salt). In other words, the "cysteine" supplements theoretically
can pick up more heavy metals in their preparation than the disulfide.
However, cysteine (alias thiol- or reduced form, -SH, or sulfhydryl) may
provide more protection against aflatoxin and other carcinogenic and toxic
mycotoxins that contaminate our food supply by reacting with these carcinogenic
substances before they are absorbed. In the case of cysteine, this probably
first requires autoxidation to its sulfenic acid, explaining the
low
rate of reaction between cysteine and aflatoxin and how cysteine can
be slowly depleted from our food supply by contaminating mycotoxins.
Since the monooxygenase is unstable in
the absence of NADPH and NADP+, a deficiency of niacin (from which
these cofactors are made) may be particularly disruptive to this enzyme
and its ability to down-regulate HMG-CoA reductase. Also, if results in
the "test tube" are any indication, the absence of oxygen should lead to
dramatic losses of the monooxygenase when
NADPH is adequate. In other words, under hypoxic (or low oxygen) conditions
thought to exist in the center of rapidly growing tumors, NADPH may aggravate
the regulatory problems caused by the loss of monooxygenase activity by
directly favoring activities of HMG-CoA reductase. When excessive, this
unregulated mevalonate synthesis can contribute to heart disease (high
cholesterol) and to tumor proliferation (isoprenylation reactions). These
relationships provide rational explanations for two previously puzzling
phenomena:
-
Depriving tumor cells of oxygen in culture
is known to make them more malignant (intractable) when inoculated into
laboratory animals.
-
Despite the fact that niacin is used to make the NADPH cofactor for the
reductase, this vitamin is better known for its ability to lower, not increase,
cholesterol.
With these considerations, niacin should be most useful in the prevention
of cancer and heart disease, especially in
tobacco users. If rapid tumor growth can be controlled by other means
and if the tumor tissue can be adequately oxygenated, niacin may have some
therapeutic value even in large tumors. There is similarly guarded therapeutic
potential in advanced heart disease, as well, since arteries occluded by
proliferation of the endothelium lead to poorly oxygenated tissues, just
as rapidly growing large tumors outstrip their oxygenated blood supply.
Because of similarly linked difficulties in these two diseases, pathological
proliferation and poor aeration of tissues, several complementary and
even alternative therapies for the vitaletheine modulators are of interest,
including hyperbaric oxygen, gene therapy increasing expression of the
monooxygenase in poorly oxygenated tissues, and proliferation-suppressing
agents that might have a sparing effect upon revascularization and reoxygenation
of tissues.
GO TO:
