Abstract
Experimental location data indicate that electrons
are not distributed as ‘balloons-of-electron-dots’ depict
for spdf atomic orbitals. Spectral data
constancies have always indicated that electrons behave quite orderly.
Consequentially, atomic orbital models with center-concentrated dots misleadingly
give
a wrong impression of
electron behavior randomness and should be nixed. Likewise, the idea of molecular bonds as
capsules filled with dots of ‘non-repelling, spin-paired electrons” should
also be nixed.
The Spherical Cloud Model of Electron
Orbitals
Orbitals are intended to provide a handle on how electrons might be arranged
around a nucleus. The data presented by Stodolna, et al[1]
using a ‘quantum microscope’ technique indicate that electrons are not
randomly dispersed. They suggest
that their results might indicate “circular or spherical” orbitals for the
hydrogen atom. While one might wonder about some indu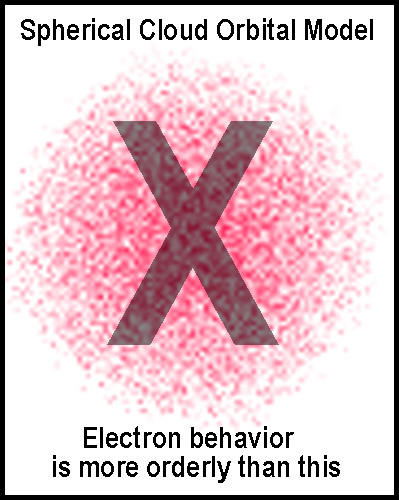 ced
artifacts, it is clear from their images that hydrogen orbitals are fairly well
defined. “Dot-matrix” cloud representations[2]
of electron orbitals, as depicted in the figure at the right, and their hybrid
mixings are not and are, thus, misleading. Such representations are presumably
efforts to convey probability distributions that are based loosely on static (no
time factor) continuum calculations. They provide no information about how or
why an electron got to a possible location or where it would go next. Speaking
of an electron as a cloud and then saying the cloud is densest where that
electron can be found is doublespeak!
ced
artifacts, it is clear from their images that hydrogen orbitals are fairly well
defined. “Dot-matrix” cloud representations[2]
of electron orbitals, as depicted in the figure at the right, and their hybrid
mixings are not and are, thus, misleading. Such representations are presumably
efforts to convey probability distributions that are based loosely on static (no
time factor) continuum calculations. They provide no information about how or
why an electron got to a possible location or where it would go next. Speaking
of an electron as a cloud and then saying the cloud is densest where that
electron can be found is doublespeak!
The ‘quantum microscope’ experimental
data, also discussed further below, show that electrons
behavior is quite orderly. Indeed, the constancy and sharpness of spectral
data have ALWAYS indicated this.
It is strange that the electron in a cloud model, which might be in any
position, low near the nucleus or far from it, nevertheless jumps in response to
the exact same photon into one precisely higher orbital position, regardless of
the point from which it jumps. Unless there are significant qualifications to
the observed experimental location and spectral data,
“electrons-can-be-anywhere-and-everywhere” probability models make no sense!
Thus, models such as that depicted in the figure above should be nixed!
Electron Orbitals as Generated by the
MCAS Model
While the authors of the ‘quantum
microscope’ data indicated that their results might indicate
“circular/spherical” orbitals for the hydrogen atom, the data also supports
non-spherical orbital shapes. Actually, the data may only represent the
summation of the outer (zenith and nadir) limits of electron movement at each
energy level.[3]
These are the points of zero movement from the nucleus where the electron is
most likely to be observed. Indeed, electrons are observed as particles whose
“roundness” and dipole character are now being sought![4]
Could they actually be too pudgy to be quantum changelings? “Electron-spin predestined the
predominant singular twist of natural molecules (e.g., DNA). With a singular
spin, electrons flow chirally around nuclei. Thus, electronic orbitals possess
built-in chirality. Atoms of the universe were the first to have a one-way
traffic system.”[5]
Orbitals should be considered as defining where electrons travel alone on beaten
“paths”, ruts, tubular conduit worm-holes or as in sync groups possibly like
“necklace” beads on an orbital “string” wave. While it may be easier to
draw orbitals with conical lobes, the actual paths may be more like twisted
paddles. Spin-pairing occurs in the MCAS model with electrons flow in opposing
orbitals in contrast to the current electron-spin reversal requirement.
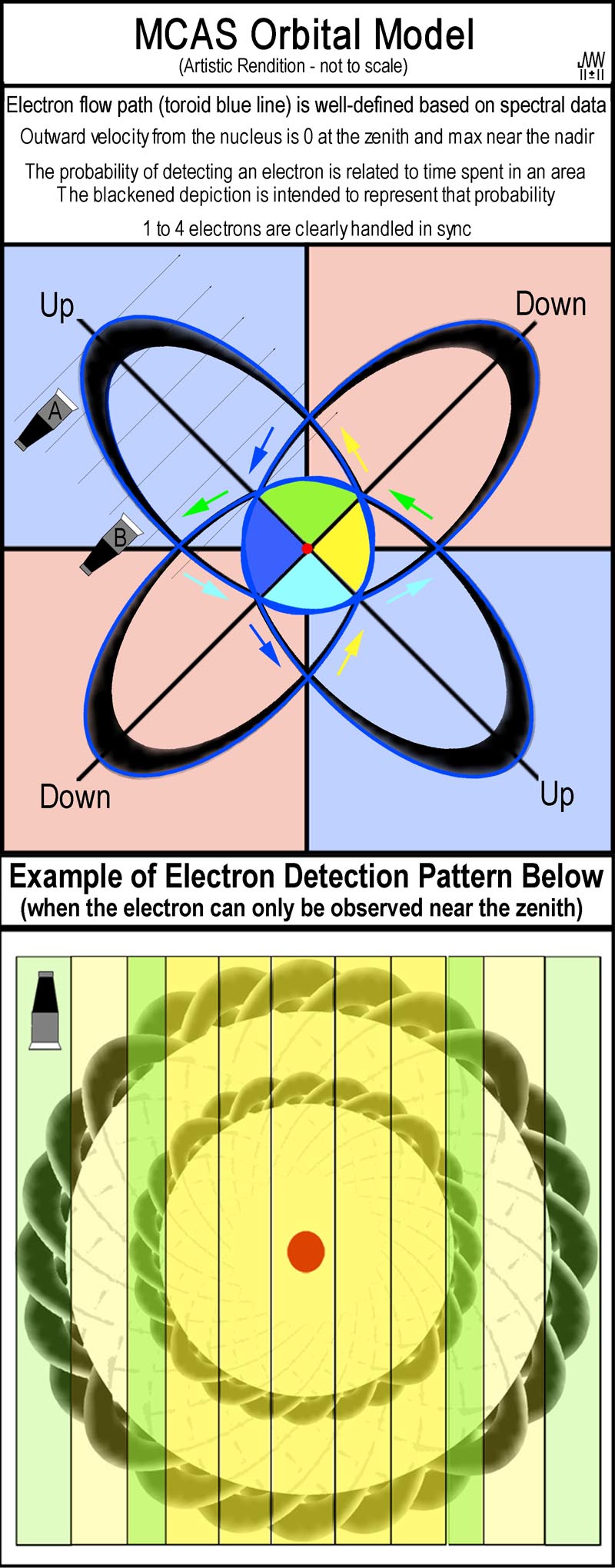 The
following discussion demonstrates how electrons will be observed per the MCAS
model. The figure at the right is a flattened MC tetrahedral orbital which the
model would use for a hydrogen atom’s simplest levels. The depiction is “not
to-scale”. The lobes in the blue quadrants were “Up”, while those in the
pink quadrants were “Down”. Electron movement will be along very narrow
paths as illustrated by the blue-lined hypotrochoid. The lobe dimensions are set by
the energy of the electrons and their electrostatic interaction with the
nucleus. The extent of the lobes is integer-based (n2 = 1, 4, 9,
etc); a simple demonstration of why this is so has been presented elsewhere[6].
Black areas inside the blue orbits are included to provide a rough indication of
the probability of an electron being observed. At the orbital zenith, the
electron is neither moving from nor towards the nucleus. Just before and
afterwards, it is also slow in that regard; elsewhere, movement to/from the
nucleus is quite rapid. Detecting an electron depends on the observing
device’s response time and sensitivity. While detection of an electron may be
obtained with “sensor A”, the same setting on “sensor B” (or a single
sensor viewing the entirety) may show nothing as an electron speeds past its
view. The situation becomes similar to the image in the figure at the lower
right when many ‘sightings’ are made and the tetrahedral orbital’s 3D
movement is not anchored along with that of the nucleus. The vertical green
strips indicate the perpendicular view that shows where the slowest movement
to/from the nucleus occurs and is thus the most likely region for success in
detecting electrons. The yellow strip areas are relatively similar to one
another and provide much less chance of detecting an electron. With sufficient
data merging, details become blurred and smooth rings are generated.
Experimental electron detections will be for 3D tetrahedral orbitals; for
illustrative purposes, the lobes are shown flattened here.
The
following discussion demonstrates how electrons will be observed per the MCAS
model. The figure at the right is a flattened MC tetrahedral orbital which the
model would use for a hydrogen atom’s simplest levels. The depiction is “not
to-scale”. The lobes in the blue quadrants were “Up”, while those in the
pink quadrants were “Down”. Electron movement will be along very narrow
paths as illustrated by the blue-lined hypotrochoid. The lobe dimensions are set by
the energy of the electrons and their electrostatic interaction with the
nucleus. The extent of the lobes is integer-based (n2 = 1, 4, 9,
etc); a simple demonstration of why this is so has been presented elsewhere[6].
Black areas inside the blue orbits are included to provide a rough indication of
the probability of an electron being observed. At the orbital zenith, the
electron is neither moving from nor towards the nucleus. Just before and
afterwards, it is also slow in that regard; elsewhere, movement to/from the
nucleus is quite rapid. Detecting an electron depends on the observing
device’s response time and sensitivity. While detection of an electron may be
obtained with “sensor A”, the same setting on “sensor B” (or a single
sensor viewing the entirety) may show nothing as an electron speeds past its
view. The situation becomes similar to the image in the figure at the lower
right when many ‘sightings’ are made and the tetrahedral orbital’s 3D
movement is not anchored along with that of the nucleus. The vertical green
strips indicate the perpendicular view that shows where the slowest movement
to/from the nucleus occurs and is thus the most likely region for success in
detecting electrons. The yellow strip areas are relatively similar to one
another and provide much less chance of detecting an electron. With sufficient
data merging, details become blurred and smooth rings are generated.
Experimental electron detections will be for 3D tetrahedral orbitals; for
illustrative purposes, the lobes are shown flattened here.
For a hydrogen atom, a single, particulate,
electron can ONLY be at one place at any given moment in time.
A second orbital level is shown to illustrate the cumulative effects of
data summing. Depending on conditions, inter orbitals, which are of lower
energy, could very well have a higher temporal concentration of that electron
and thus show up more intense than outer ones.
Conclusion:
the probability of an electron being found at a location depends on temporal
as well as non-temporal factors, such as electrostatic interactions and
energy levels.
The
MCAS Model and ‘Quantum Microscope’ Results
In order to evaluate how the MCAS model
fits the ‘quantum microscope’ results1
, the images from that work have been inspected. That work is very
elaborate and extensive. Holding the nucleus in a rigid location is quite
impressive as is the detection of an electron’s position. In the end, however,
the accomplished effort simply provides a collection of singular results:
projected 2D locations of the 3D locations of a single electron, in the case of
a hydrogen atom, regardless of how the electron got there. Impressive numbers of
singular results were required to indicate that the electron spends time in
well-defined “orbitals”.
The figure
below has been created to interpret the ‘quantum microscope’ results and to
relate the MCAS atomic orbital model to them.
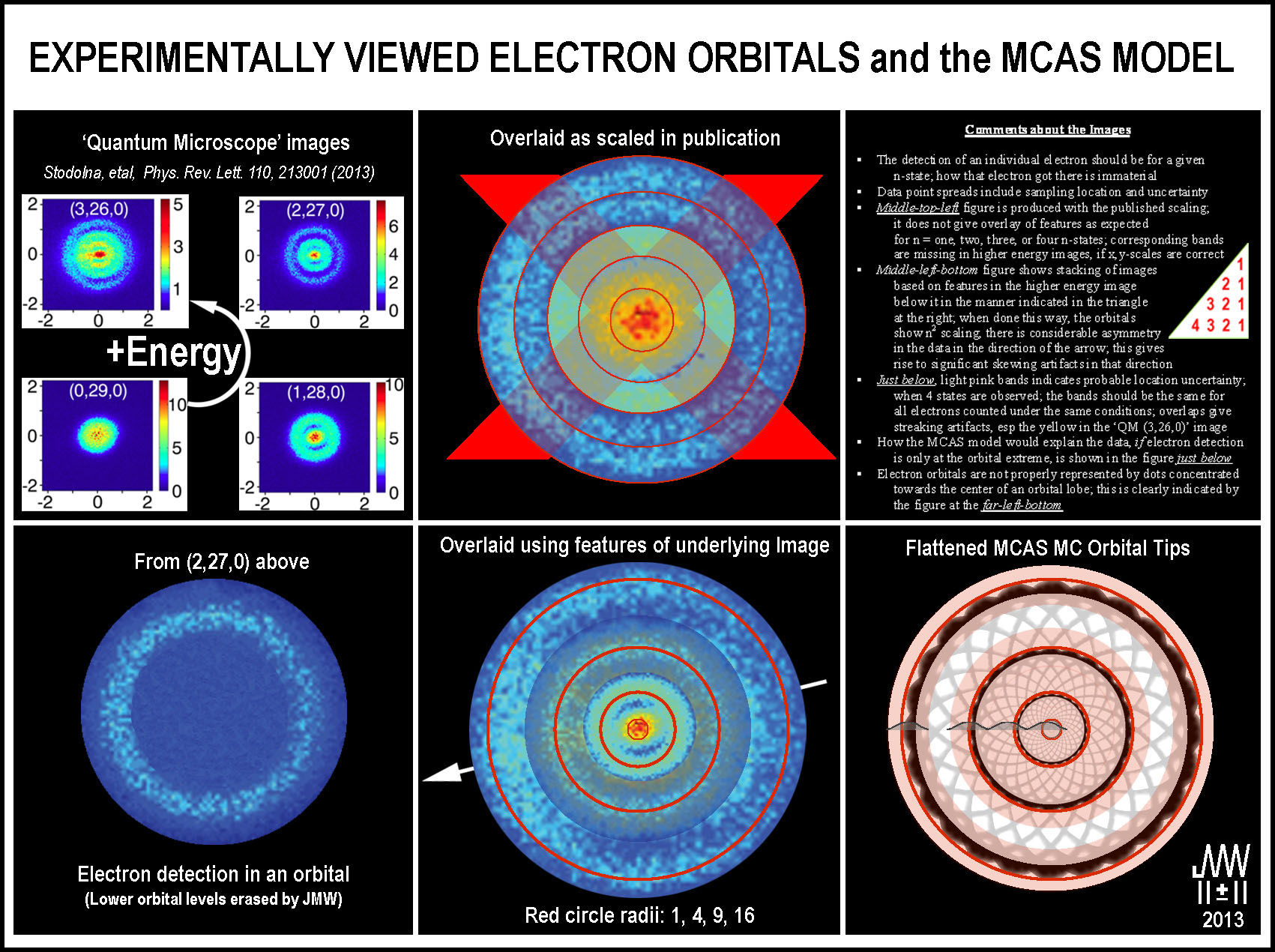
The original images (upper-left set of
images) demonstrate cleanly separated, outer regions for the higher
activated states. Delving into the inner orbital makeup is a bit difficult when
the images are apart. To address this problem, the images, which were indicated
in the published work to have the same scale, were stacked with the highest
energy image at the bottom (upper-middle
image). The outer region of each lower energy image was removed in order not
to obscure that portion of the image below. Since the second lowest energy image
indicated a tight center, that center was copied and placed on the top.
Alternating rings would have to be missing in several of the published images,
if the individual x-y scales are correct. Also, some image details, esp. those
of the middle two energy level images, do not overlay. This overlay from the
publication’s indicated scaling is inconsistent and therefore has been “X’d” out.
The published images are stacked
differently in the bottom-middle image.
Each lower energy image is scaled to the inner structure of the higher energy
one placed just below in the manner indicated in the triangle in the upper-right
image. Again, the outer portion of the next upper image is removed to show
the outer region of the image below. Images higher in the stack have also been
made more transparent to show some of the lower image’s details. While the
rings are nearly circular, they are not uniform. An obvious bias (asymmetry in
the detection or detector array?) in the populations is indicated by the arrow.
This bias is likely a systematic issue in the data collection as it appears in
all of images. The spread in the rings is likely from uncertainties in both
actual electron locations and experimental error. Together, they will produce
the artifacts that prevent the “dark blue” circular regions that separate
the lower energy orbitals from being complete and the non-circular skewing in
the ‘QM (0,29,0)’ image. More about this is discussed below.
The PhysRevLett article indicates that only
four levels are present and that is clearly evident in the bottom-middle
image. It is also clear that the levels scale closely to n2 = 12,
22, 32, and 42 (see the red circles in the bottom-middle image) when stacked in this manner.
There is considerable asymmetry in the data
in the direction of the arrow. This gives rise to significant skewing artifacts
in that direction. The light pink circular bands in the lower-right
image indicate the possible uncertainty in the characterization of the
highest energy orbital of the ‘QM (3,26,0)’ image. It would be the same for
all of the orbitals measured under the same conditions. (The second highest
energy image (‘QM (2,27,0)’) appears to have a bit less uncertainty which
would explain why it is sharper and more widely shown.) The uncertainty bands
overlap for the lowest 3 energy orbital states in the ‘QM (3,26,0)’ image;
this leads to streaking artifacts, notably, the yellow ones.
How the MCAS model would explain the data, if
electron detection is only at the orbital extreme, is shown in the lower-right-image. The tip portions of the flattened MC-orbitals are
placed at the mid-region of the various energy rings. This indicates zero
movement towards or from the nucleus. While the electron will move through
3D-space in its journey to and from the nucleus, it is at these zeniths that the
electron will most readily be detected. The probability of locating an electron
has a time factor as well as the normal electrostatic ones that are typically
considered.
The lower-left image shows an experimentally observed orbital – the
outer orbital of the ‘QM (2,27,0)’ image – wherein the lower energy
orbitals have been erased for clarity. This image clearly demonstrates that
electron orbitals are not properly represented by dots concentrated towards the
center of an orbital lobe. Dot representations are even more misleading when the
lobes are not even nucleus-centered! One should also seriously question the
imagery of molecular bonds as “capsules” whose space is filled with dots of
‘electrostatically non-repelling, spin-paired, electrons’.
REFERENCES
[1]
A. S. Stodolna, A. Rouzée, F. Lépine, S. Cohen, F. Robicheaux,
A. Gijsbertsen, J. H. Jungmann, C. Bordas, and M. J. J. Vrakking, Hydrogen
Atoms under Magnification: Direct Observation of the Nodal Structure of
Stark States, Phys. Rev. Lett. 110, 213001 (2013)
[4]
H. Loh, K, etal., “Precision spectroscopy of polarized molecules in an ion
trap”. Science (2013) Vol. 342 no.
6163 pp. 1220-1222; editor’s
summary: “One of the signatures of this “new physics” would be a
non-vanishing electric dipole moment of the electron - http://www.sciencemag.org/content/342/6163/1220.abstract
[5]
Joel M Williams, “The Electronic
Structure of Atoms”, in Challenging Science, AuthorHouse, 2005, p15
 - click
- click 

 ced
artifacts, it is clear from their images that hydrogen orbitals are fairly well
defined. “Dot-matrix” cloud representations
ced
artifacts, it is clear from their images that hydrogen orbitals are fairly well
defined. “Dot-matrix” cloud representations The
following discussion demonstrates how electrons will be observed per the MCAS
model. The figure at the right is a flattened MC tetrahedral orbital which the
model would use for a hydrogen atom’s simplest levels. The depiction is “not
to-scale”. The lobes in the blue quadrants were “Up”, while those in the
pink quadrants were “Down”. Electron movement will be along very narrow
paths as illustrated by the blue-lined hypotrochoid. The lobe dimensions are set by
the energy of the electrons and their electrostatic interaction with the
nucleus. The extent of the lobes is integer-based (n2 = 1, 4, 9,
etc); a simple demonstration of why this is so has been presented elsewhere
The
following discussion demonstrates how electrons will be observed per the MCAS
model. The figure at the right is a flattened MC tetrahedral orbital which the
model would use for a hydrogen atom’s simplest levels. The depiction is “not
to-scale”. The lobes in the blue quadrants were “Up”, while those in the
pink quadrants were “Down”. Electron movement will be along very narrow
paths as illustrated by the blue-lined hypotrochoid. The lobe dimensions are set by
the energy of the electrons and their electrostatic interaction with the
nucleus. The extent of the lobes is integer-based (n2 = 1, 4, 9,
etc); a simple demonstration of why this is so has been presented elsewhere