|
 | |||||||||||||
|
||||||||||||||
|
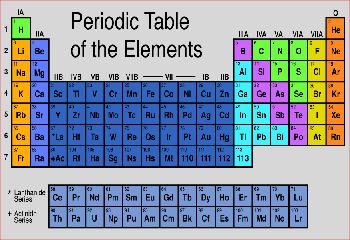
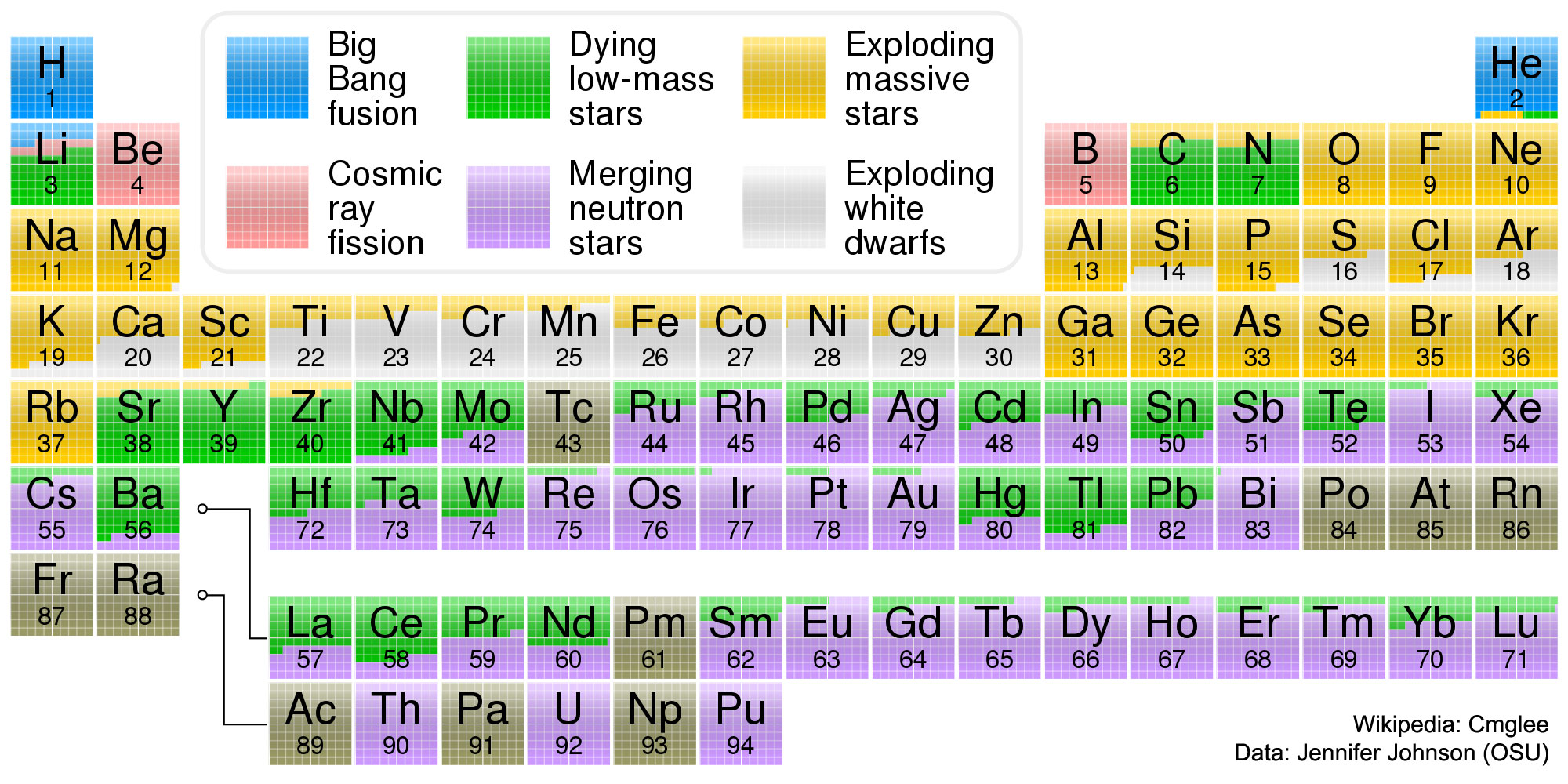
I tried to sketch the state of knowledge about elements chemistry and alchemy in the mid-1880's. Many elements and their properties were known by then, an many small patterns had been discovered. I started out with a long strip of paper, with all the elements on it, on 4"×4" boxes - this is a loooong strip. I used colors to highlight the elements that were known at the time, and correspondences in chemical properties that were known by the 1850's: Sodium, Potassium and Lithium behaved similarly, Aluminum, Indium, Tin and other sets. If you line these sets up below each other, you can roll up the strip several times. What Mendeleev and Alexandre-Emile Béguyer de Chancourtois did was to line them up in a table, such that element's properties periodically repeat in columns. I got the scissors out, and a roll of tape, and turned the ribbon of elements into the table. Once the table was in place, the hunt was on for elements in the empty places in the table - moreover, the table tells you the properties of these elements. The realization that all elements had a place in this table was a great leap forward in science, and it qickly led to more discoveries and understanding.
Links:
|
||||||||||||||

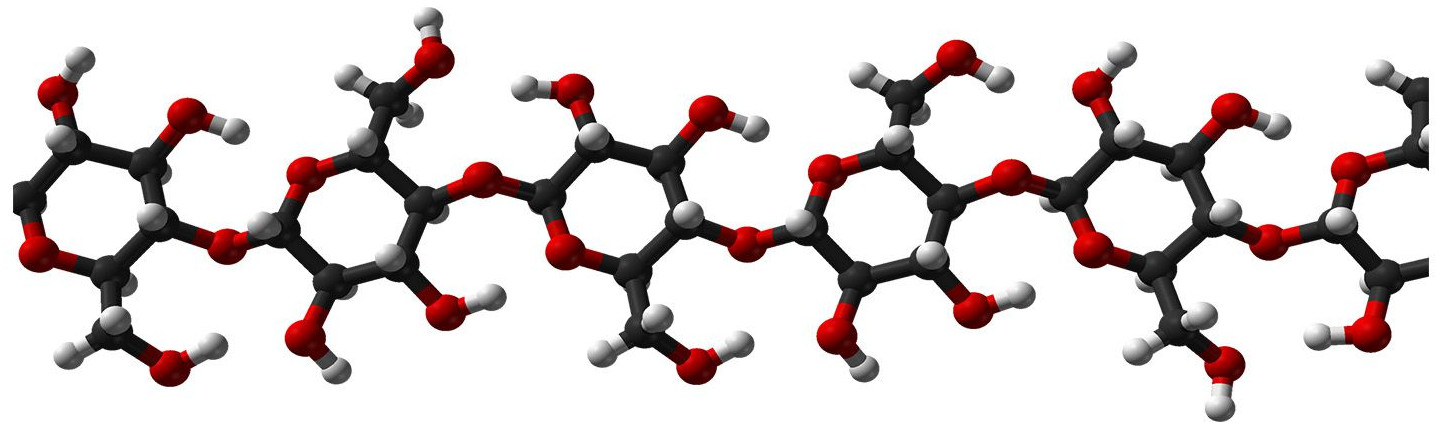
Chemical structure
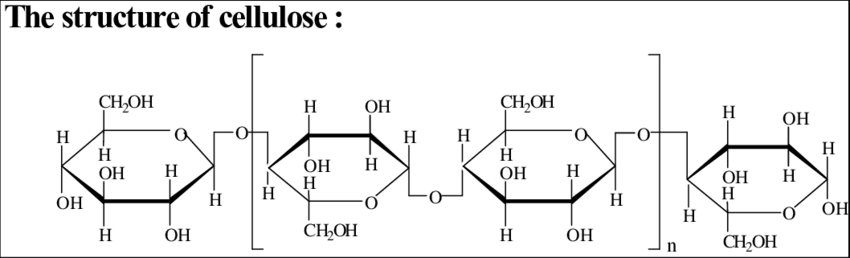
In 2024, I had a set of questions about the periodic table (above), and there were other questions about larger molecules, DNA, and chemistry-related items. So I did a unit starting with the periodic table, then how to build bigger and bigger molecules, and pointing out that carbon is the only atom out of all the elements that allows you to build bigger and bigger molecule, up to DNA. Then I extracted DNA from strawberries.
Some links:
|
||||||||||||||
 How do computers work?
How do computers work? First, as always, a little history: I brought in my small collection of old computer stuff: a vacuum tube, a transistor, a deck of punch cards, paper tape, 9" and 5" floppy disks, a giant few-Mbyte hard disk etc. etc. A story goes with each, since I have been working with computers since the days of punchcards. We did a bit of binary addition on the board, and spelled 'Hello' in binary ascii code. Then I took a small desktop computer apart, and showed all the bits, pretty much as in the video linked below.
Links:
|
||||||||||||||
|
Ms. Yanda had an article from National Geographic (March 2008) about the 'god particle' that she was sharing with her class. Since I had worked at CERN in the 80's and 90's, I took an hour to talk about CERN, the LHC accelerator, detectors and the discoveries that may be right around the corner (including possibly the production of (harmless) black holes). I connected with the Periodic Table story from two weeks earlier, where the discovery of an orderly pattern led directly to the discovery of elements that had not been seen yet, and a far deeper understanding of what gave rise to all this regularity. Similarly, with the current 'periodic table' of elementary particles (quarks and leptons), there is one more particle that supposedly exists, but has not been seen yet: the Higgs, named after Peter Higgs, pictures on the right. LHC turns on in less than 2 months! In the local newspaper, an article had appeared just a few days earlier about this.
Links:
|
||||||||||||||
|
What caused the ice ages and can it happen again? First, how can we tell that there were ice ages in the past? There signs of glaciers of long-gone glaciers on the ground across the continents, from New York west across the continent, and across northern europe, eurasia to the pacific coast. Second, you can read the history in ice cores, sediment cores from the ocean bottom, and you can read the underground temperatures down boreholes. All can reveal temperatures of years past. They show that in the last half-million we've had a series of warm periods, much like the one we ae in now, alr=ternating with periods that were much colder - the ice ages. The ice ages last about
Links:
|
||||||||||||||
| ||||||||||||||