Copyright © 1996, 1997, 2001 by Galen Daryl
Knight and VitaleTherapeutics, Inc.
Aflatoxins and Carcinogenesis Through Alkylation of Vitaletheine Modulators?
The following analyses provide a cohesive theory for explaining the carcinogenic
potential of a variety of microbial and chemical toxins. The decrease in
life expectancy for people residing in the US circa de 1993 indicates
that there may be a resurgence of these problems brought on by an industrialization
of our society and our food supply, including the warehousing of foodstuffs
for strategic reserves and price controls. A return to a more "Victory
Garden" mentality and the consumption of more raw foodstuffs may do more
for our general health than medicines currently being developed. From this
discussion, it appears that if one is saturated with any combinations of
industrial, myco- and/or afla- toxins that destroy the vitaletheine modulators,
then the potential for positive intervention with these promising new immunostimulants
is severely compromised. Because of this, control of our environment and
proper nutrition (prophylaxis) should receive as much emphasis for maintaining
good health as is placed upon medical care.
Aflatoxin in Liver Cancer
Although there are
individual researchers
who are not convinced that aflatoxin is a carcinogen in man, the recent
realization of just how important the vitaletheine modulators are to the
immune system's ability to fight off cancer indicates otherwise. It is
true that infection with hepatitis B virus seems to have a stronger correlation
with the incidence of liver cancer than does exposure to aflatoxins in
our foodstuffs. However, suppression of vitaletheine modulator-mediated
immune responses by aflatoxin consumed in contaminated food by the general
population may have a profound effect upon the epidemiology of hepatitis
B viral infections, as well as upon risks for other types of infectious
disease such as hantavirus, ebola, tuberculosis, cancer, AIDS, etc.. In
this regard it is probably critical to the general health of the public
that aflatoxins, known to be carcinogenic in laboratory animals, be minimized
in our diets, especially when the biosynthesis of the vitaletheine modulators
are otherwise compromised by dietary deficiencies and by exposure to other
environmental toxins and carcinogens.
Probable Reactions of Carcinogenic Aflatoxins with the Vitaletheine Modulators
Aflatoxins can undergo many of the same reactions with sulfenic acids characteristic
of reactions with dimedone or vitamin C.
Adjacent keto- groups make any hydrogen on the intervening carbon acidic,
favoring tautomerization of this structure to an enol. Like the enol tautomer
of dimedone, these probably react with sulfenic acids to alkylate the sulfur,
thereby generating a sulfide-linked adduct.
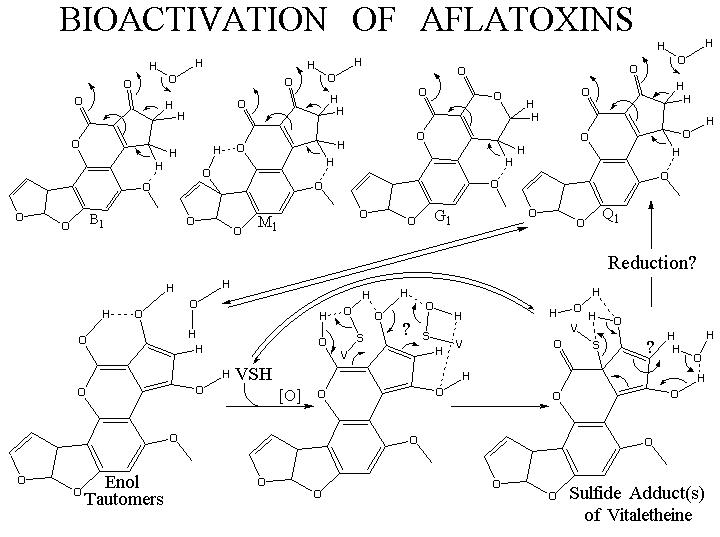
Possible Roles of Aflatoxins in a Variety of Diseases
These sulfides in turn can be oxidized in reactions catalyzed by monooxygenases,
an activity that may contribute further to an uncoupling of the vitaletheine
modulator/monooxygenase control of immunological responses. In this regard,
it is interesting to note that the
control
of cholesterol feedback regulation studied by Siperstein also is lost,
not only in spontaneous mouse and human liver tumors, but also in hepatoma
caused by aflatoxin. This suggests that both high cholesterol (heart disease)
and liver cancer have a common contributing factor in aflatoxin, a link
that may be described in part by the
observed
interactions between HMG-CoA reductase and the monooxygenase thought
to be the target for the vitaletheine modulators.
Among the aflatoxins, the structure of Q1 presents some interesting
chemistry that may explain its relatively low carcinogenic potential. Because
it is hydroxylated at a position in conjugation with the most likely site
for thioalkylation with vitaletheine, this aflatoxin may be able to rearrange
and spontaneously liberate vitaletheine so trapped. As a consequence of
this, aflatoxin Q1 would be expected to be less carcinogenic than B1, just
as ascorbate is less toxic than dimedone. Using a similar comparison, it
is also interesting that dehydroascorbate
is diabetogenic, hinting that thiols may form reversible adducts with
the more oxidized versions of these bis-keto- structures. This last reaction,
of course, would be subject to equilibration with other, more abundant
thiols in the poisoned cells, such as glutathione and metallothionine that
provide some protection for the vitaletheine modulators. The toxicity of
dehydroascorbate is yet another reason to avoid high doses of vitamin C,
since saturation of mechanisms for rereducing vitamin C that has autoxidized
could lead to this and other mechanistically-related health problems. Some
toxicity is still expected for the aflatoxin, Q1, since it appears to have
some capacity to trap a second sulfenic acid (at the indicated position,
"?"), and this reaction should effectively disrupt the conjugation responsible
for the spontaneous liberation of trapped vitaletheine.
The seriousness of this interference of aflatoxin with sulfenic acid
metabolism is illustrated by known effects of sulfenic acid formation,
and the reaction of this sulfenic acid with dimedone, upon one of the key
enzymes for energy production. Formation of a
sulfenic
acid on glyceraldehyde-3-phosphate dehydrogenase changes this energy-producing,
enzyme system into an acyl phosphatase activity, essentially uncoupling
the pathway for producing energy from sugar. Upon reaction of the sulfenic
acid with dimedone, both activities of this enzyme are unrecoverably lost.
This is very similar to an observed
monooxygenase-dependent
irreversible inactivation of HMG-CoA reductase with dimedone. Since
the affected site in the dehydrogenase/acyl phosphatase seems to be a cysteine
residue, aflatoxin (reacting in a manner similar to dimedone) could seriously
compromise, not only the production of energy by this pathway, but also
the availability of cysteine. This problem in turn compromises the biosynthesis
and availability of the vitaletheine modulators. It would be interesting
to determine if the AIDS virus, itself, or opportunistic infections of
organisms capable of producing bis-keto- compounds (such as the Aspergillus
flavus that produces aflatoxins) results in the observed symptomatology,
i.e., low energy and weight loss, low plasma cysteine levels, and immunosuppression
through these types of mechanisms.
Other Carcinogenic Mycotoxins that can React with Vitaletheine Modulators
There is already considerable evidence that mycotoxins produced by microorganisms
in our foodstuffs constitute a serious threat to human health through likely
reactions of their enols and epoxides with the vitaletheine modulators.
Enol tautomers can be formed in ochratoxin A, patulin, and citrinin produced
by Aspergillus-Penicillium. Trichothecens and zearalenone produced
by Fusarium species are also capable of producing enol tautomers.
The trichothecenes and vomitoxin, and the chemical warfare agent, nivalenol,
in addition to having the capacity for enol tautomerism, have
epoxide
groups capable of reacting directly with the thiol forms of the vitaletheine
modulators, as does T-2 and fusarenon X. Epoxides are also contained in
the toxins, rioridin A, verracurol, verrucarin A, and muconomycin A. The
profound effects that these compounds have upon immunity has been extensively
reviewed by
James J. Pestka and Genevieve
S. Bondy.
Small-Molecular-Weight Carcinogenic Chemicals that can React with the Vitaletheine
Modulators
While these mycotoxins tend to be rather complex organic molecules, there
is evidence that even the simple epitopes within these compounds are carcinogenic.
Consequently, these simple compounds should be eliminated from our industrialized
societies whenever and wherever possible.
Proprionolactones,
ethylene imines, and epoxides have all been identified as carcinogenic
substances that react with thiols. Incubations with thiols, or the amino
acid cysteine, inactivate the carcinogenic potential of many of these substances,
confirming what is probably a sparing effect upon the vitaletheine modulators.
Although direct reactions with the thiol moieties of the vitaletheine modulators
can account for most of the observed toxicities of the compounds in this
particular article, mechanisms involving the monooxygenase system must
be evoked to explain the toxicities of a few of those mentioned.
Analysis of Carcinogenic Potential Based Upon Oxidation States
According to the above-referenced article, maleic anhydride reacts with
cysteine and produces tumors in rats, but sodium maleate is not carcinogenic.
Likewise, the thiol reagent, N-ethylmaleimide, is not carcinogenic. The
"thiol" reagent, N-ethylmaleimide, probably reacts immediately with glutathione
in the cell that serves to protect any vitaletheine modulators that might
happen to be in the thiol form. The vitaletheine modulators are expected
to be mostly in their oxidized forms due to reactions driven by the monooxygenase,
so sulfenamides are more likely products of reactions of such amines with
the vitaletheine modulators. Sulfenamides formed in this manner can be
easily hydrolyzed, for example, to N-hydroxy-maleimide and the corresponding
free thiols. The carcinogenic maleic anhydride, on the other hand, may
undergo an initial reaction of thiol with its carbonyl-conjugated alkene
to form a sulfide. The resulting sulfide and enol tautomer then can react
with a sulfenic acid moiety, say of the vitaletheine modulator family,
to form a bis-sulfide adduct. According to the above scenario, glutathione
might activate the carcinogenic potential of maleic anhydride, while sulfenic
acids of the vitaletheine modulator family would preferentially react with
this enol-tautomer-containing sulfide product. An adduct containing a sulfide
of each, one glutathione and one vitaletheine moiety, is expected. Also
consistent with this theory is the observation that succinic anhydride,
lacking the carbonyl conjugation found in maleic anhydride, is not carcinogenic.
Reaction of succinic anhydride with thiols should produce only a thiol
ester, probably that of glutathione, that is easily hydrolyzed by endogenous
thiolesterases to regenerate the free thiol. Consequently, succinic anhydride
is far less likely than maleic anhydride to react with the sulfenic acids
of the vitaletheine modulators, perhaps accounting for the observed differences
in the carcinogenic potential for these two substances.
This theory, that compounds capable of reacting with sulfenic acids
to produce stable sulfides are carcinogenic through their ability to alkylate
the sulfur of the vitaletheine modulators, finds some support in the well-known
tumor-promoting activities of
phorbol esters.
GO TO:

