Abstract
There is increasing evidence that the
hydrogen atom in X—H—X
connections is covalently bound to both X atoms in equal to varying degrees. A
spherical s-orbital for hydrogen attached to two 2-electron covalent bonds
clearly violates the spdf-QM concept of shared electron bonding. A short
discussion of the situation is presented along with a simple alternative
orbital model for hydrogen that allows hydrogen to have two, diametrically
opposite, covalent bonds.
INTRODUCTION
Molecule interactions form our physical world. While
solids indicate strong interactions between atoms and within molecules, e.g.
crystals and polymers, water is the epitome of “loose” interactions. As
such, water has been one of the most studied substances, both as object and
solvent. Martin Chaplin has assembled a website with 2065 references entitled
“Water Structure and Science”[1].
Since this paper will be about
hydrogen bonding in O—H—O systems, the following figure is presented to give
the reader a feel for the atomic distances. The figure is adapted from Chaplin1
. Information presented in the Data
Appendix at the end of this paper has been added to provide perspective.
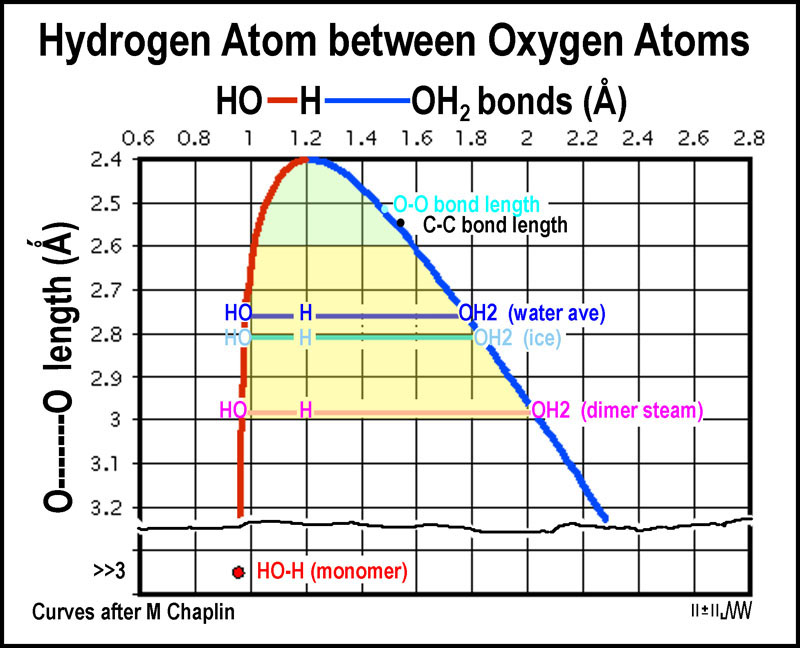 The
figure clearly indicates the long bond that is associated with the term
“hydrogen bonding” that allows H2O to exist as water. The same
bond in ice is just a bit longer. Surprisingly, the bond is much longer in the H2O
dimer that occurs about 1 in 1000 water molecules and about 1 in 20 steam
molecules[1]
. Chaplin indicates that the hydrogen bonding strength is
15kJ/mole at a length of 2.99Å. The light yellow zone reflects that an extended
covalent bond is well within this distance.
The
figure clearly indicates the long bond that is associated with the term
“hydrogen bonding” that allows H2O to exist as water. The same
bond in ice is just a bit longer. Surprisingly, the bond is much longer in the H2O
dimer that occurs about 1 in 1000 water molecules and about 1 in 20 steam
molecules[1]
. Chaplin indicates that the hydrogen bonding strength is
15kJ/mole at a length of 2.99Å. The light yellow zone reflects that an extended
covalent bond is well within this distance.
Chaplin covers a number of
articles dealing with the covalence of hydrogen bonds. A few of his comments
(mainly from footnote ‘d’[1]
) are presented below to give the reader a feel for the situation.
The reader should refer to Chaplin’s
website for references to each of these comments.
- The (water)JMW
network is essentially complete at ambient temperatures; that is, (almost)
all molecules are linked by at least one unbroken hydrogen bonded pathway.
- If the water
hydrogen bond (the long one)JMW
is considered within the context of the complete range of molecular hydrogen
bonding then it appears most probable that it is not solely electrostatic.
- If the hydrogen
bond is substantially bent, it follows that the bond strength is weaker.
- The ionization
of water, the continuous transformation of ice VII to ice X and the lower
ionization potential for liquid water relative to water vapor would all seem
to indicate a continuity of electron sharing between water molecules.
- In nucleic
acids, inter-nucleotide N-H····N coupling (2JNN, using 15N nuclei)
confirms some covalent nature in the N-H····N hydrogen bond.
- 3-bond NMR
(3JNC) splitting has been found through peptide N-H····O=C hydrogen
bonds in proteins, confirming some covalent nature in the N-H····O
hydrogen bonds.
- Hydrogen bonds
in other molecules, such as DNA, also possess considerable covalent
character.
“Hydrogen bonding” is
commonly associated with long, weak, O-----H interactions. When the oxygen atoms
are closer, the hydrogen nucleus is more equally distant from each oxygen
nucleus (the light green area in the figure above). Such short distances would
be expected for proton-transfers from one oxygen atom to another.
Proton-transfer might be a fleeting event, but the interaction of the oxygen
orbitals with the hydrogen would be instantaneous with the hydrogen atom having
two short covalent bonds. How can this occur when, in the current spdf-QM model,
hydrogen has only a spherical s-orbital and by definition can only form a single
covalent bond having 2 electrons?
A
stable molecular arrangement with two oxygen atoms sharing a hydrogen atom
equally at short range would definitely require the hydrogen to have two
covalent bonds. Do such molecular
arrangements exist? The answer is “Yes”.
DISCUSSION
Dimethysulfoxide solvated proton
(DMSO—H—OSDM)+ is a stable molecular arrangement with a
symmetrically placed proton between the two oxygen atoms (O—H—O). This
molecular species was first reported by Williams and Kreevoy in 1967 when we
studied methanesulfonic acid in DMSO.[2]
A similar conclusion was drawn by Kirilova, etal.[3]
in their 1986 study with ATR. No bands belonging to (DMSO—H—OSDM)+
other than v(O—H—O) were found. X-ray studies have shown the occurrence of (DMSO—H—OSDM)+
in crystalline salts.[4]
In 2002, Denisov, etal.[5],
determined the cation to be practically linear with a O—O distance of
2.403Å with the bridging proton located exactly in the center between the two
oxygen atoms.
In their 2005 study of crystalline H5O2+ClO4-,
Vener and Sauer[6]
found the equilibrium O—O distances to be 2.431Å in the isolated cation and
2.426Å in the crystal.
The covalent orbital reach of an
oxygen atom is greater than 0.74Å (½ of O—O) and that of a hydrogen atom is
greater than 0.37Å (½ of H—H). The
full reach of each atom to the other will be greater than this 1.11Å sum. It is
reasonable to expect them to span the 1.2Å distance of O—H bonds easily. In
each of the molecular arrangements above, the hydrogen atom is thus clearly
involved with two covalent bonds which, in the current spdf-QM bonding model,
require it to be coordinated with 4 electrons.
The current spdf-QM electron
orbital model is a forced one based on the precept of a sphere as the starting
point. The spdf-QM model not only requires that macro-physical laws, such as e-e
repulsion, cease in the near-nucleus area, but has a basic set of orbitals with
little resemblance to those needed to model the materials of our substantive
world. To address how atoms actually bond to one another, the orbitals of
spdf-QM model had to be “hybridized”. For the simplest orbital connections,
sp3, sp2 and sp1 orbital sets were created.
Eventually, when inorganic complexes were modeled more “hybridizing” was
needed with d-orbitals becoming involved. Interestingly, with all of this
“hybridizing” of orbitals, the spherical s-orbital of hydrogen was
consciously excluded (avoided?). In the bond formation part of the currently
accepted spdf-QM hybridized model, bonds contain 2 paired electrons.
Consequently, hydrogen is able to form only 1 covalent bond as its unhybridized
orbital can only handle 2 electrons. From the real-world cases above, a hydrogen
atom does form more than one covalent bond is necessary. Clearly, the spherical
s-orbital for hydrogen is incorrect for such bonding. It is even incorrect for a
solo hydrogen atom and a helium atom[7].
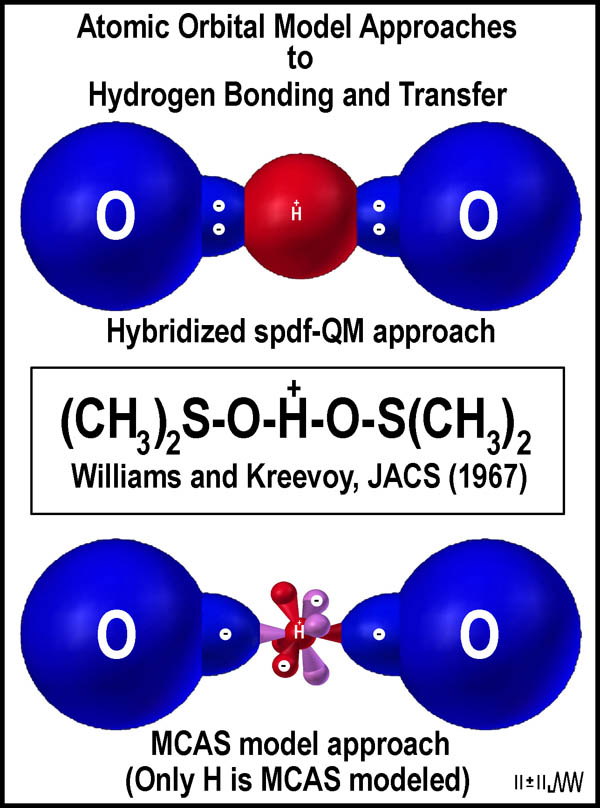 The
upper image in the figure at the right indicates the problem with the current
spdf-QM spherical hydrogen orbital. Two identical covalent bonds are needed for
the O—H—O connection. Each oxygen atom (only one of their orbital
protuberances is shown) supplies 2 electrons, but the hydrogen atom is able to
form only one 2-electron bond. Thus, two covalent bonds being attached to the
hydrogen atom violates a prime tenet of the spdf-QM orbital approach to
molecular bonding.
The
upper image in the figure at the right indicates the problem with the current
spdf-QM spherical hydrogen orbital. Two identical covalent bonds are needed for
the O—H—O connection. Each oxygen atom (only one of their orbital
protuberances is shown) supplies 2 electrons, but the hydrogen atom is able to
form only one 2-electron bond. Thus, two covalent bonds being attached to the
hydrogen atom violates a prime tenet of the spdf-QM orbital approach to
molecular bonding.
The MCAS model[8]
was created as the result of the need for hydrogen atoms to have two covalent
bonds as well as to eliminate the need for spin-reversed electron pairing.
“Mirrored tetrahedral orbital twins” (center of the bottom image in the
figure at the right) provide precisely the needed orbital configuration for
hydrogen. The oxygen atoms are not MCAS-modeled here (shown as spheres with an
orbital protuberance), so that the focus is on the MCAS character of the
hydrogen orbitals; their orbitals, too, are “mirrored orbital twins”.
Electrons are not corralled in the covalent bond, but flow through the orbital
sets. The mirrored hydrogen orbital pair establishes an opposing linear
orientation of the covalent bonds from the hydrogen atom.
The MCAS model negates the need to “hybridize” an atom’s orbitals for the
simplest bonding between atoms! Dogged adherence of the scientific community to
Bohr’s orb-turned-sphere for the hydrogen atom has stifled the search for
physical model alternatives to the spdf-QM atomic model. Strong pi-bonds from
parallel p-orbitals that can hardly touch, and do so at an acute angle, counter
experimental evidence that bonds become progressive weaker as they deviate from
linearity. Molecular orbital diagrams remove the physical aspects of the
orbitals. In essence, MOs simply indicate that bonds are formed without worrying
about how electrons in them actually move or reside. How and where electrons
move in the atomic and molecular systems is the aim of the MCAS model. A
spherical orbital with spin-reversed paired electron twins in highly localized,
interatomic, bond space is not part of this model.
DATA
APPENDIX
From Martin Chaplin, Water
Structure and Science, http://www1.lsbu.ac.uk/water/hbond.html
and http://www1.lsbu.ac.uk/water/molecule.html
o
The experimental values for gaseous
water molecule O—H length is 0.95718 Å
o
The O—O distance in ice Ih varies
between 2.75 Å (0 K) and 2.764 Å (253 K).
o
In ambient atmosphere the O—O in
the water dimer is 2.985 Å (calculated by JMW);
the short O—H bond is 0.948Å and the long bond is 2.037Å
From U.
Bergmann, A.Di Cicco, P. Wernet, E. Principi, P. Glatzel, and A. Nilsson, Nearest-neighbor
oxygen distances in liquid water and ice observed by x-ray Raman based extended
x-ray absorption fine structure,
J Chem Phys. 2007 Nov 7;127(17):174504, http://www.ncbi.nlm.nih.gov/pubmed/17994824
o
O—O of water average distance
(2.81 Å for water and 2.76 Å for ice)
o
And a slightly shorter peak
position (2.70 Å for water and 2.71 Å for ice)
From C Chieh, http://www.science.uwaterloo.ca/~cchieh/cact/c120/bondel.html
o
C—C 1.54 Å
o
O—O 1.48 Å
o
H—H 0.74 Å
REFERENCES
[1]
Martin
Chaplin, Water
Structure and Science, http://www1.lsbu.ac.uk/water/hbond.html
[2]
J M Williams and M M Kreevoy,
Structure and
infrared spectrum of the solvated proton in dimethyl sulfoxide,
J. Am. Chem. Soc., 1967, 89 (21),
pp 5499–5501
[3]
A.P. Kirilova, V.D.
Mayorov, A.I. Serebryanskaya, N.B. Librovich, E.N. Guriyanova, Izvest. Akad.
Nauk
USSR
, Ser. Khirn. 10 (1986) 2435
(cited in ref 5 below)
[4]
References cited in
ref 5 below: R.A.
Potts, Inorg. Chern. 9 (1970)
1284; B.R. James, R.H. Morris, JACS Chem. Commun. (1980) 31; O.V.
Rudnitskaja, T.M. Buslaeva, N.I. Lyalina, Zh. Neorg. Khim. 39
(1994) 922; and V.I. Lobadyuk, V.N. Spevak, N.K. SkVortsov, A.I. Stash, V.K.
Belskij, Zh. Obshch. Khim. 66
(1996) 705.
[5]
G.S. Denisov, A. Koll, V.I.
Lobadyuk, V.M. Schreiber, A.V. Shurukhina, V.N. Spevak,
Hydrogen bonding
in coordination compounds containing homoconjugated bis-dimethylsulfoxide
cation, J Molecular Structure 605 (2002) 221-226; http://www.chemie.fu-berlin.de/~limbach/denisov/184.pdf
[6]
V. Vener and J Sauer,
Environmental effects on vibrational proton dynamics in H5O2+:
DFT study on crystalline H5O2+ClO4-,
Phys . Chem. Chem. Phys . , 2005, 7
, 258-263; http://edoc.hu-berlin.de/oa/articles/recUjCiEZgtpY/PDF/247whSivydg.pdf
[7]
Joel M Williams, Electron
orbitals for ortho and para
Helium, http://pages.swcp.com/~jmw-mcw/electron
orbitals for ortho and para helium.htm; a pdf version is available at http://gsjournal.net/Science-Journals/Essays/View/4980;
He (11±11) is a gas.
[8]
A description of the MCAS atomic model is presented along with the
spdf-QM atomic model in this reference: Joel M Williams, Parsing
the spdf electron orbital model, http://pages.swcp.com/~jmw-mcw/Parsing
the spdf electron orbital model.htm;
a pdf version is available at http://gsjournal.net/Science-Journals/Essays/View/5032
![]()


 The
figure clearly indicates the long bond that is associated with the term
“hydrogen bonding” that allows H2O to exist as water. The same
bond in ice is just a bit longer. Surprisingly, the bond is much longer in the H2O
dimer that occurs about 1 in 1000 water molecules and about 1 in 20 steam
molecules[
The
figure clearly indicates the long bond that is associated with the term
“hydrogen bonding” that allows H2O to exist as water. The same
bond in ice is just a bit longer. Surprisingly, the bond is much longer in the H2O
dimer that occurs about 1 in 1000 water molecules and about 1 in 20 steam
molecules[ The
upper image in the figure at the right indicates the problem with the current
spdf-QM spherical hydrogen orbital. Two identical covalent bonds are needed for
the O—H—O connection. Each oxygen atom (only one of their orbital
protuberances is shown) supplies 2 electrons, but the hydrogen atom is able to
form only one 2-electron bond. Thus, two covalent bonds being attached to the
hydrogen atom violates a prime tenet of the spdf-QM orbital approach to
molecular bonding.
The
upper image in the figure at the right indicates the problem with the current
spdf-QM spherical hydrogen orbital. Two identical covalent bonds are needed for
the O—H—O connection. Each oxygen atom (only one of their orbital
protuberances is shown) supplies 2 electrons, but the hydrogen atom is able to
form only one 2-electron bond. Thus, two covalent bonds being attached to the
hydrogen atom violates a prime tenet of the spdf-QM orbital approach to
molecular bonding.