
This article and others are included in the 18 chapters of
a 164 page paperback compilation of articles by JMW
$19.95
US list at Amazon
Click
here for Amazon.com
By Joel M Williams (text and images
©2014)
For a free pdf file of the webpage at the General Science Journal click here

|
This article and others are included in the 18 chapters of a 164 page paperback compilation of articles by JMW $19.95
US list at Amazon
|
Abstract
The bonding of the hydrogen atom in the B—H—B sequence of diborane
is just a standard overlapping of atomic orbitals. With the MCAS model,
hydrogen naturally has the capacity to form linear and non-linear bonds
between two atoms when necessary. There is no need for the nebulous 3-center,
two-electron, bond that has been used to honor the unassailable 1s-orbital of
the spdf-QM model.
INTRODUCTION
Hydrogen atoms bonded to something singularly glided along smoothly. Hydrogen bonding in things like water was glossed over as not a real molecular bond, but an electrostatic addition to its real covalent bond. That a proton might have two “real” molecular bonds when placed between some oxygen atoms hardly caused a ripple, although it should have.
When chemists began studying
boron chemistry, multiple molecular bonding of hydrogen to boron atoms became a
more onerous problem. Did the scientific world “hybridize” hydrogen as it
had carbon to convert s+3p’s to 4sp3’s? Nope. The
“holiest-of-holies” was unassailable. Thus was born the 3-center,
two-electron, bond.
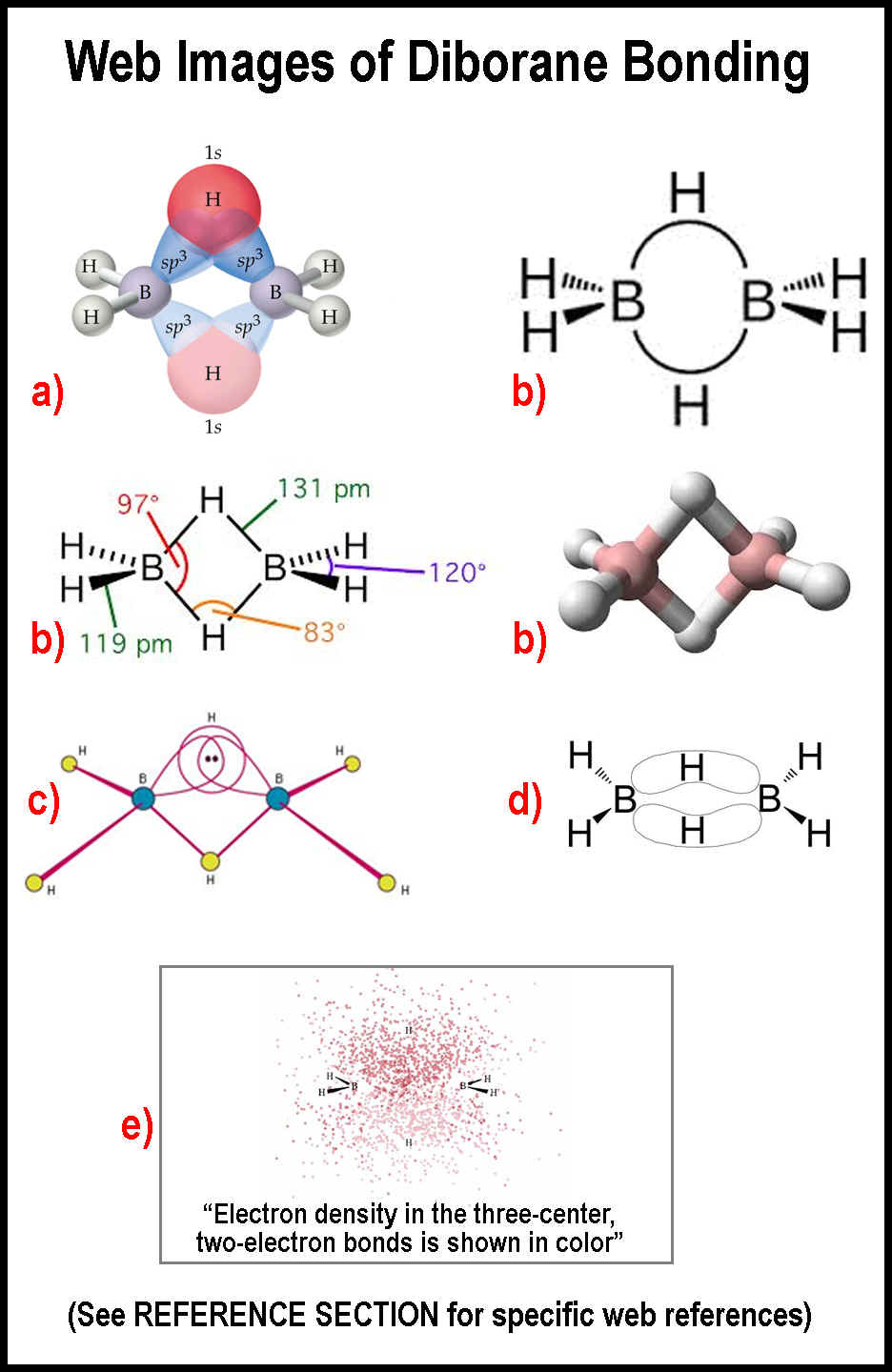 The
figure at the right contains a sampling of some of the images that appear on the
web to portray the B—H—B, 3-center, two-electron, bond. The red letters
correspond to those in reference [1]
at the end of this paper.
The upper left image a indicates that a spherical hydrogen bonds with two sp3
hybrid orbitals. Just how is unclear; it just does. The upper right image
b indicates that there are B-to-B bonds, just what those
bonds are is not clear, and that somehow hydrogen gets involved. The left middle
image
b gives physical data and indicates two bonds for the
middle hydrogen atoms, but orbital types are not indicated; a 122-degree bond angle
instead of the 120-degree angle is listed elsewhere.[2]
The middle right image
b makes the hydrogen atoms appear a bit like an oxygen atom
. The next lower left image
c
presumably indicated what a 3-center, two-electron, bond is; well, it shows 3
orbitals at a juncture. How those orbitals interact is not clear. This is
especially problematic now that electrons are being detected as particles
flowing in orbits.[3]
The next lower right image
d looks like there is a pi-cloud between the boron atoms
and somehow the hydrogen atoms are afloat in it. Experimental evidence shows
that electrons really are particulate at the nuclear level, however.[3]
The very bottom image
e takes the cake in my estimation and shows what happens when
folks get carried away with those dot matrix representations of orbitals![3]
The website
for e claims that this is “the current picture of bonding in
diborane”. You have to wonder what students are learning from this sort of
imagery. Little information about the bonding mechanics of the hydrogen atoms is
forthcoming from these images, except for the physical location of the hydrogen
atoms and that two of the hydrogen atoms ARE each attached to two boron atoms,
contrary to the spdf-QM precept that hydrogen forms single, 2-electron, bonds!
The
figure at the right contains a sampling of some of the images that appear on the
web to portray the B—H—B, 3-center, two-electron, bond. The red letters
correspond to those in reference [1]
at the end of this paper.
The upper left image a indicates that a spherical hydrogen bonds with two sp3
hybrid orbitals. Just how is unclear; it just does. The upper right image
b indicates that there are B-to-B bonds, just what those
bonds are is not clear, and that somehow hydrogen gets involved. The left middle
image
b gives physical data and indicates two bonds for the
middle hydrogen atoms, but orbital types are not indicated; a 122-degree bond angle
instead of the 120-degree angle is listed elsewhere.[2]
The middle right image
b makes the hydrogen atoms appear a bit like an oxygen atom
. The next lower left image
c
presumably indicated what a 3-center, two-electron, bond is; well, it shows 3
orbitals at a juncture. How those orbitals interact is not clear. This is
especially problematic now that electrons are being detected as particles
flowing in orbits.[3]
The next lower right image
d looks like there is a pi-cloud between the boron atoms
and somehow the hydrogen atoms are afloat in it. Experimental evidence shows
that electrons really are particulate at the nuclear level, however.[3]
The very bottom image
e takes the cake in my estimation and shows what happens when
folks get carried away with those dot matrix representations of orbitals![3]
The website
for e claims that this is “the current picture of bonding in
diborane”. You have to wonder what students are learning from this sort of
imagery. Little information about the bonding mechanics of the hydrogen atoms is
forthcoming from these images, except for the physical location of the hydrogen
atoms and that two of the hydrogen atoms ARE each attached to two boron atoms,
contrary to the spdf-QM precept that hydrogen forms single, 2-electron, bonds!
 While I was studying
supercritical fluids,[4]
it became clear to me that hydrogen atoms should not have orbitals that differed
from those of other atoms and certainly not spherical ones. Indeed, WHY should
those of hydrogen be different? So, I sent a submission to the academic press in
1993 indicating that hydrogen should have a tetrahedral orbital rather than a
spherical one.[5]
This was the birth of the MCAS atomic orbital model.
While I was studying
supercritical fluids,[4]
it became clear to me that hydrogen atoms should not have orbitals that differed
from those of other atoms and certainly not spherical ones. Indeed, WHY should
those of hydrogen be different? So, I sent a submission to the academic press in
1993 indicating that hydrogen should have a tetrahedral orbital rather than a
spherical one.[5]
This was the birth of the MCAS atomic orbital model.
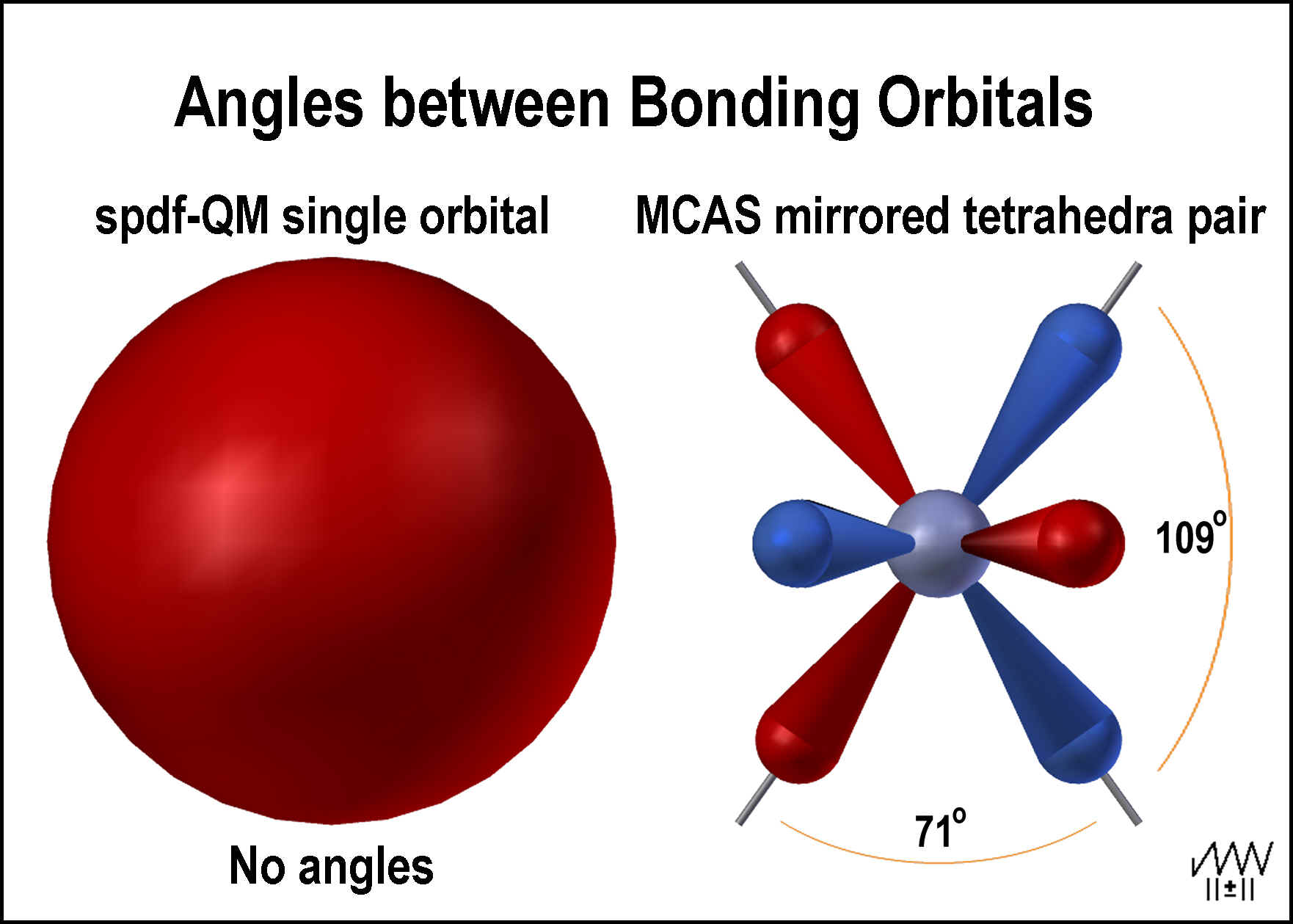
The figure at the right contrasts the spherical orbital of the spdf-QM model with the mirrored tetrahedral pair of the MCAS model. What differentiates hydrogen and helium from other elements is the tightness (extent) of the first energy level. At the first energy level, each tetrahedral unit (red or blue here) is dimensionally too small to handle more than a single electron. Thus, hydrogen and helium are the only elements of the first period of the periodic table. As orbital extents increase with higher energy levels, the orbitals contain one electron each at first (pairing) and then three more each until there are eight. Thus, a periodicity of eight occurs in the outer energy level as it does in the periodic table. Creating the periodic table via the MCAS model is presented elsewhere.[6] The first level orbitals of hydrogen (and helium) need to be no different than those of any other element![7]
MULTIPLE
BONDING OF HYDROGEN ATOMS
 Having pointed out that the MCAS
atomic orbitals are the same for all atoms, multiple bonds for hydrogen atoms
should not be strange at all. The main reason that they are not common results
from the fact that a single electron in the non-bonding tetrahedral set is
electrostatically sufficient for the singly positive proton. The diametrically
opposed tetrahedral sets of the MCAS model signal the logic of linear X—H—X
bonds, however. Such linear arrangements are found for protons between two
oxygen atoms (O—H—O).[8]
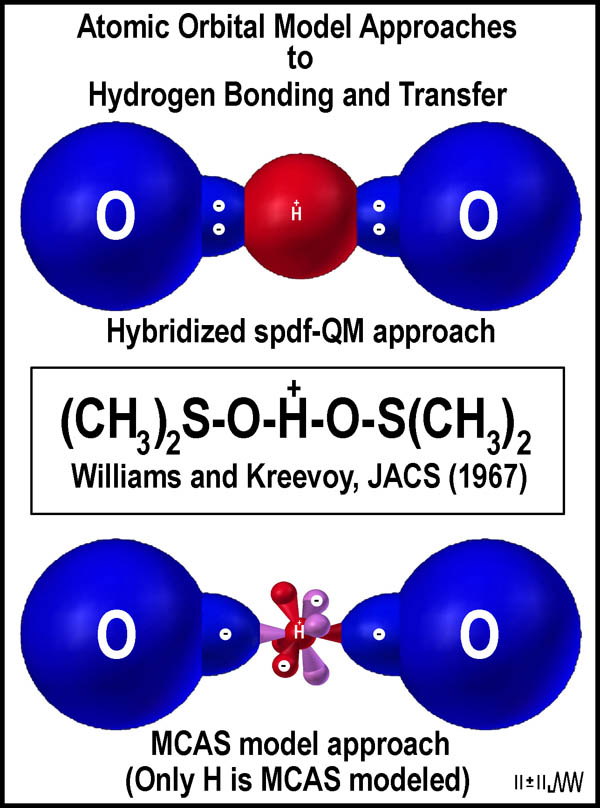
The situation for a proton in DMSO[9]
is depicted in the figure to the right. Note that 4-electrons are necessarily
involved around the hydrogen atom in the "conventional" approach. Only
two are involved in the MCAS case; further understanding of why this is so is
clear when the oxygen atoms are modeled MCAS.
Having pointed out that the MCAS
atomic orbitals are the same for all atoms, multiple bonds for hydrogen atoms
should not be strange at all. The main reason that they are not common results
from the fact that a single electron in the non-bonding tetrahedral set is
electrostatically sufficient for the singly positive proton. The diametrically
opposed tetrahedral sets of the MCAS model signal the logic of linear X—H—X
bonds, however. Such linear arrangements are found for protons between two
oxygen atoms (O—H—O).[8]
The situation for a proton in DMSO[9]
is depicted in the figure to the right. Note that 4-electrons are necessarily
involved around the hydrogen atom in the "conventional" approach. Only
two are involved in the MCAS case; further understanding of why this is so is
clear when the oxygen atoms are modeled MCAS.
Diborane presents a different problem as the B—H—B bonds are NOT linear! The figure below shows the "conventional" (3-center, 2-electron, 1s) modeling for the bridging hydrogens along with the MCAS modeling in which the bridging hydrogens have mirrored pairs of 4-orbitals (as discussed above).
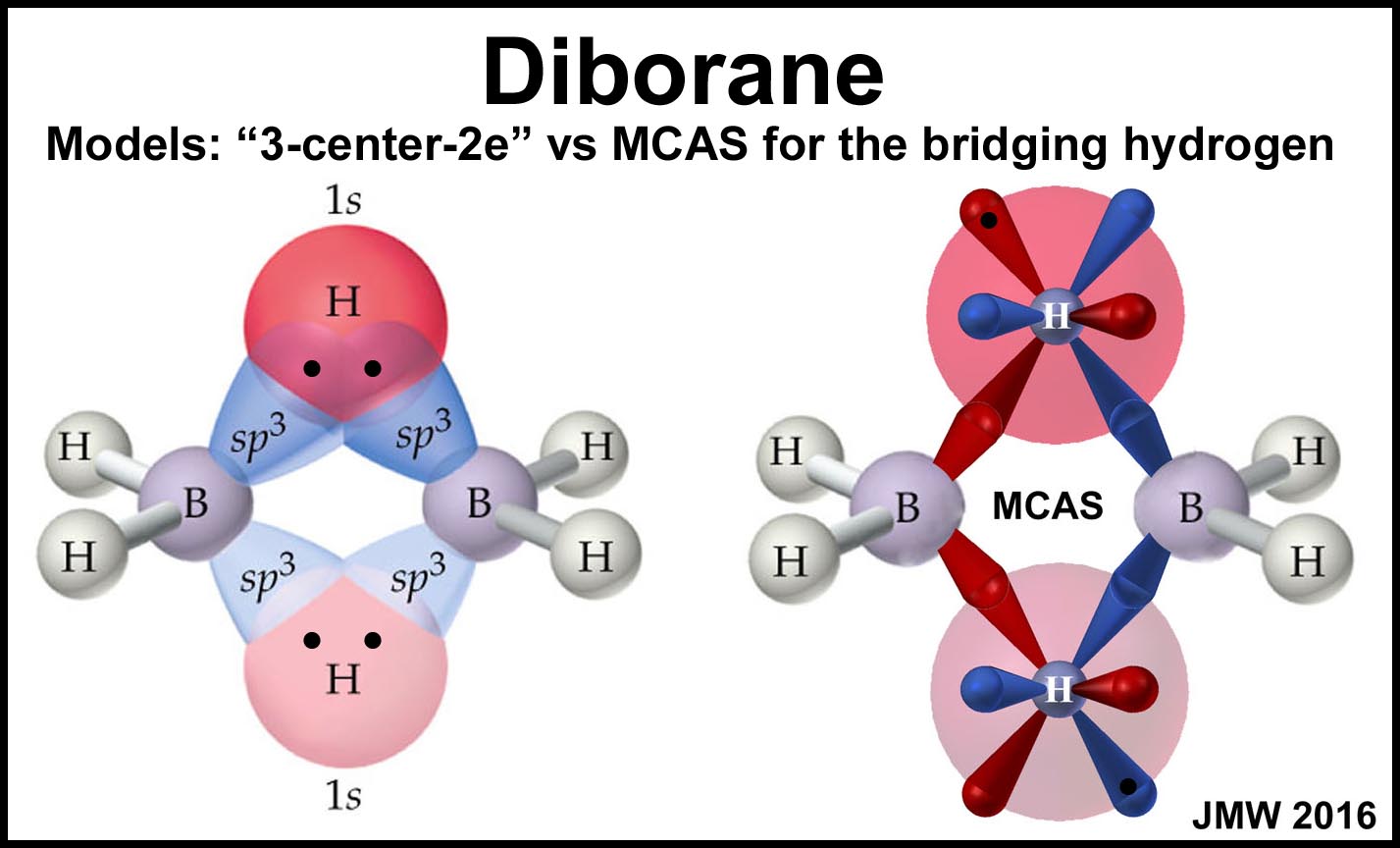
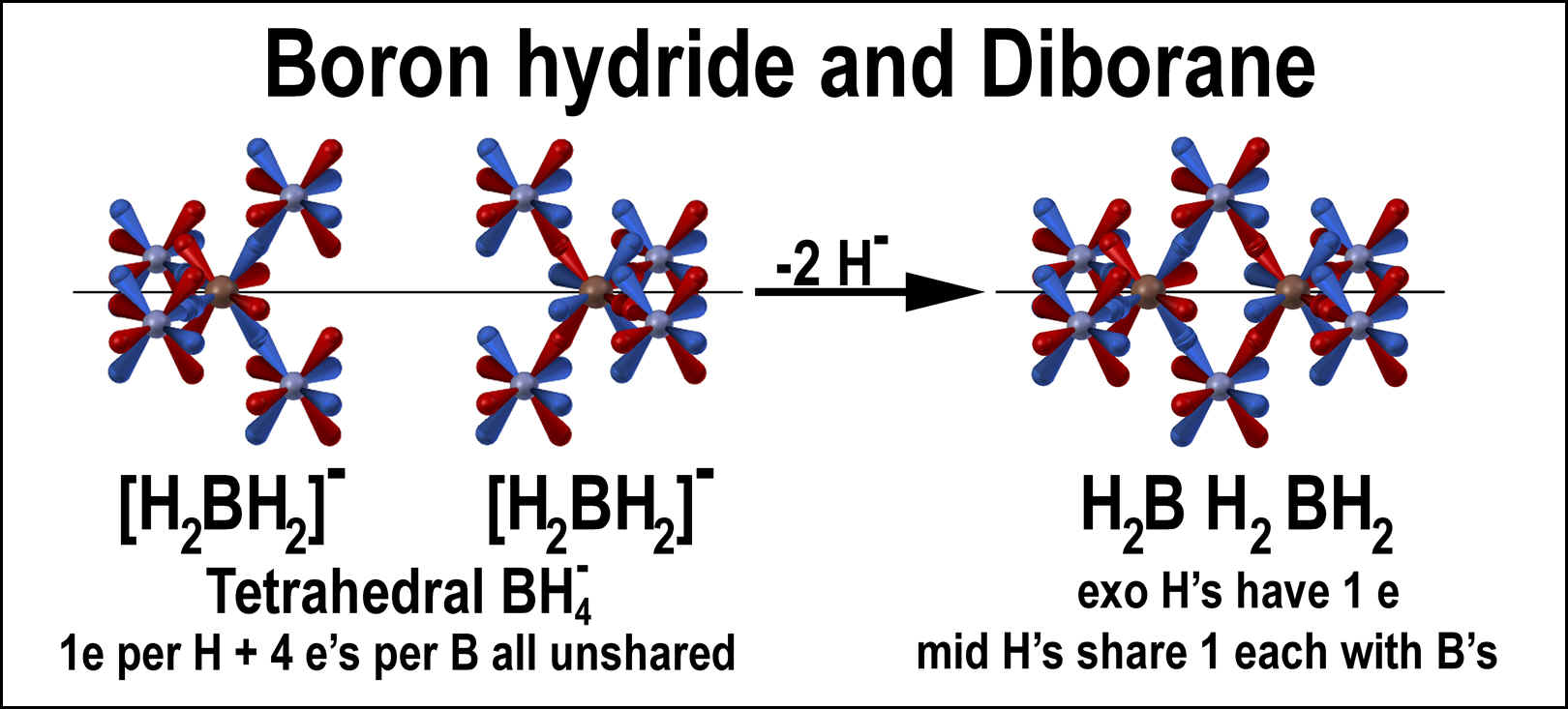
Boron hydride is a “methane analog”. Each of the four hydrogen atom is identical and surrounds the boron atom tetrahedrally. A single electron moves around each hydrogen atom in the non-overlapped (bond) orbital set. Since 4 electrons move around the boron atom in its non-bonding set, the negative charge of the boron hydride unit is centered. The images in the left of the figure below show the orbital alignments. The two boron hydride images are reverse color-coded for the purpose of demonstrating their combination to diborane.
Diborane is a molecule wherein two boron hydride anions are merged with the removal of 2 protons and 4 electrons. The figure at the right illustrates this process. The two types of hydrogen atoms differ in their connectivity. Four (4) hydrogen atoms have a normal, single orbital overlapped bond with their electron in the non-overlapped tetrahedral set and thus not shared. Two (2) hydrogen atoms have two bonds formed by the overlap of an orbital of each its tetrahedral sets with that of two different boron atoms. These atoms share electrons with each other and the boron atoms through the bond linkages. There are two different paths: one is indicated “blue” and the other “red”.
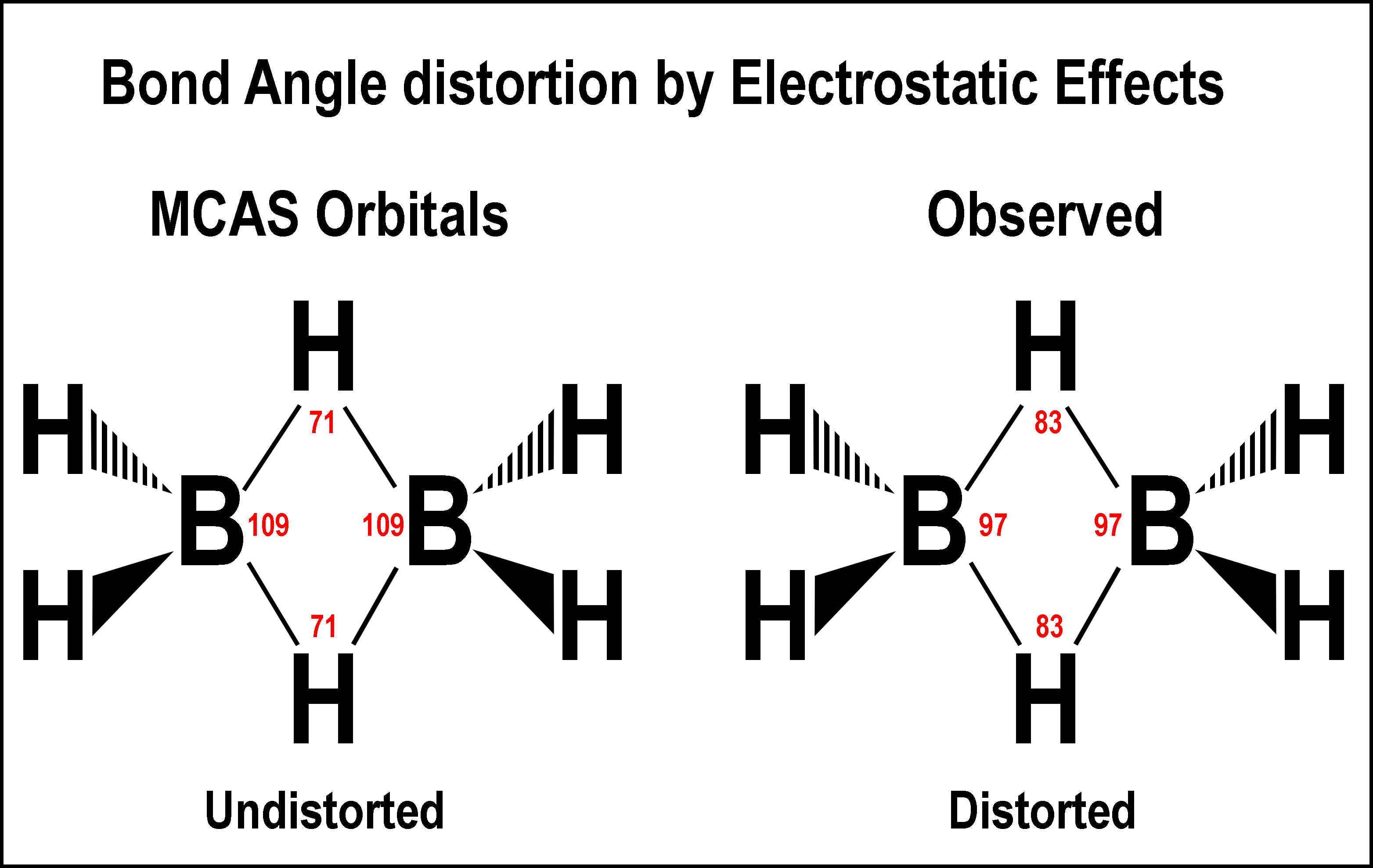 The
undistorted bond angles of the center bridge are 109º for the H—B—H
connections and 71º for the B—H—B connections. Repulsion between the boron
nuclei forces them apart and narrows the H—B—H angles while lengthen the
B—H bonds and widening the B—H—B angle. This is illustrated in the figure
at the right.
The
undistorted bond angles of the center bridge are 109º for the H—B—H
connections and 71º for the B—H—B connections. Repulsion between the boron
nuclei forces them apart and narrows the H—B—H angles while lengthen the
B—H bonds and widening the B—H—B angle. This is illustrated in the figure
at the right.
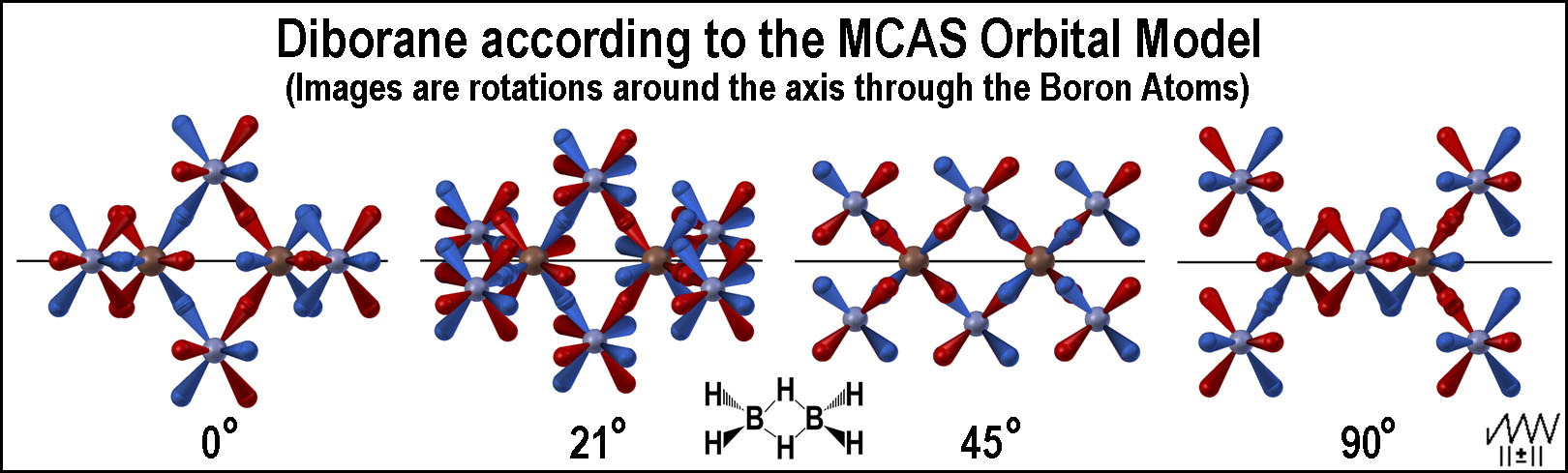
The following figure shows the undistorted orbitals of diborane as the molecule is rotated around the boron-boron nuclear axis.
One wonders, as a reader has pointed out, why linear (H2B)nH2 molecules would not occur. The main reason is that boron can form B—B bonds, too. These will promote electron flow through 3D with occasional double-bonded hydrogen atoms.
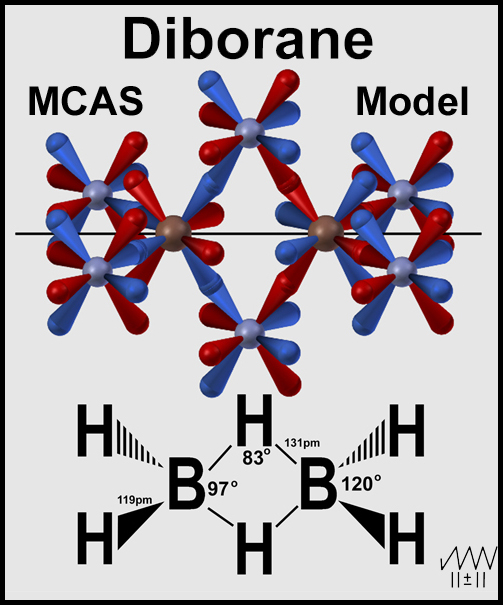
Below is diborane by the MCAS model.
CONCLUSIONS
[1] Web reference for the images of diborane in the text:
a) http://wps.prenhall.com/wps/media/objects/948/971150/ch19_04.htm
b) http://en.wikipedia.org/wiki/Diborane
c) http://www.britannica.com/EBchecked/media/956/The-structure-of-the-three-centre-two-electron-bond-in
[3]
Joel M Williams, Nixing
the ‘Balloons-of-Electron-Dots’ Atomic Orbital Models, http://pages.swcp.com/~jmw-mcw/Nixing
the 'Balloons-of-Electron-Dots Atomic Orbital Models.htm
[4]
Joel M Williams and George H. Sprenger, THE
4TH STATE OF
[5]
The submission was neither accepted nor peer-reviewed.
[6]
Joel M Williams, Creating the Familiar
Periodic Table via MCAS Electron Orbital Filling,
http://pages.swcp.com/~jmw-mcw/The
Familiar Periodic Table of Elements and Electron Orbital Filling.htm
[7]
Joel M Williams, Comparing Several
Orbital Approaches to the Hydrogen Molecule, http://pages.swcp.com/~jmw-mcw/Orbital
Models and the Hydrogen%20Molecule; and Electron
Orbitals for Ortho and Para
Helium, http://pages.swcp.com/~jmw-mcw/electron
orbitals for ortho and para helium.htm
[8] Joel M Williams, Hydrogen Bonding and Orbital Models, http://pages.swcp.com/~jmw-mcw/Hydrogen Bonding and Orbital Models.htm
[9] J M Williams and M M Kreevoy, Structure and infrared spectrum of the solvated proton in dimethyl sulfoxide, J. Am. Chem. Soc., 1967, 89 (21), pp 5499–5501